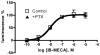Translocation of arrestin induced by human A(3) adenosine receptor ligands in an engineered cell line: comparison with G protein-dependent pathways - PubMed (original) (raw)
Comparative Study
Translocation of arrestin induced by human A(3) adenosine receptor ligands in an engineered cell line: comparison with G protein-dependent pathways
Zhan-Guo Gao et al. Pharmacol Res. 2008 Apr.
Abstract
Structurally diverse ligands were studied in A(3) adenosine receptor (AR)-mediated beta-arrestin translocation in engineered CHO cells. The agonist potency and efficacy were similar, although not identical, to their G protein signaling. However, differences have also been found. MRS542, MRS1760, and other adenosine derivatives, A(3)AR antagonists in cyclic AMP assays, were partial agonists in beta-arrestin translocation, indicating possible biased agonism. The xanthine 7-riboside DBXRM, a full agonist, was only partially efficacious in beta-arrestin translocation. DBXRM was shown to induce a lesser extent of desensitization compared with IB-MECA. In kinetic studies, MRS3558, a potent and selective A(3)AR agonist, induced beta-arrestin translocation significantly faster than IB-MECA and Cl-IB-MECA. Non-nucleoside antagonists showed similar inhibitory potencies as previously reported. PTX pretreatment completely abolished ERK1/2 activation, but not arrestin translocation. Thus, lead candidates for biased agonists at the A(3)AR have been identified with this arrestin-translocation assay, which promises to be an effective tool for ligand screening.
Figures
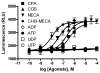
Figure 1
Concentration-response curves in the β-arrestin translocation assay for known synthetic AR pharmalogical probes (CPA 2, CGS21680 3, NECA 1, and Cl-IB-MECA 4) and the native nucleotide ligands (ADP 5, ATP 6) of various P2Y receptor subtypes. Results are expressed mean ± SEM from three separate experiments performed in duplicate.
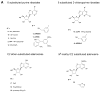
Figure 2
Selected structures of ligands that were tested in the β-arrestin translocation assay using the CHO-ADORA3 Pathhunter cell line. A. Adenosine derivatives containing the natural riboside-like 5′-CH2OH group. B. NECA-like 5′-CONH-alkyl derivatives and ring-constrained (N)-methanocarba derivatives. C. Diverse structures that were shown previously to interact with the human A3AR.
Figure 2
Selected structures of ligands that were tested in the β-arrestin translocation assay using the CHO-ADORA3 Pathhunter cell line. A. Adenosine derivatives containing the natural riboside-like 5′-CH2OH group. B. NECA-like 5′-CONH-alkyl derivatives and ring-constrained (N)-methanocarba derivatives. C. Diverse structures that were shown previously to interact with the human A3AR.
Figure 2
Selected structures of ligands that were tested in the β-arrestin translocation assay using the CHO-ADORA3 Pathhunter cell line. A. Adenosine derivatives containing the natural riboside-like 5′-CH2OH group. B. NECA-like 5′-CONH-alkyl derivatives and ring-constrained (N)-methanocarba derivatives. C. Diverse structures that were shown previously to interact with the human A3AR.
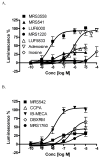
Figure 3
Concentration-response curves for structurally diverse A3AR modulators in the β-arrestin translocation assay in the CHO-ADORA3 Pathhunter cell line. Results are expressed mean ± SEM from three separate experiments performed in duplicate.
Figure 4
Kinetic analysis of four A3AR agonists in the β-arrestin translocation assay in the CHO-ADORA3 Pathhunter cell line. Results are expressed mean ± SEM from three separate experiments performed in duplicate.
Figure 5
Two nonnucleoside A3AR antagonists, the triazoloquinazoline derivative MRS1220 114 (A,C) and the pyridine derivative MRS1523 108 (B,D), characterized pharmacologically in the β-arrestin translocation assay in the CHO-ADORA3 Pathhunter cell line. Concentration response curves for the agonist Cl-IB-MECA 4 (A,B) and Schild analysis [19] of the antagonism was carried out (C,D). The Schild slopes were calculated to be 0.73 and 1.40 for C and D, respectively.
Figure 6
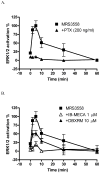
Effect of PTX pretreatment (200 ng/ml) on β-arrestin translocation induced by IB-MECA. PTX was incubated with cells for 24 h before the measurement. Results are expressed as mean ± SEM from three experiments.
Figure 7
Studies of A3AR-mediated phosphorylation of ERK1/2 in the CHO-ADORA3 Pathhunter cell line. (A) Effects of pertussis toxin (PTX) pretreatment (24 h) on MRS3558 (1 μM)-induced activation of ERK1/2, and (B) pretreatment (24 h) with nucleosides IB-MECA and DBXRM desensitize the activation of ERK1/2. In each experiment, the maximal level of ERK1/2 activation was set to 100% and that observed in unstimulated to 0%. Results are expressed as mean ± SEM (n=3).
Figure 8
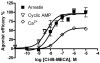
Comparison of the potency and efficacy of Cl-IB-MECA 4 at various A3AR-mediated signaling pathways. NECA was used a full agonist in all assays. The efficacy of NECA in each assay was expressed as 100%. Results are expressed as mean ± SEM of 3–5 separate experiments performed in duplicate or triplicate. The potency and Emax values from arrestin and cyclic AMP assays were listed in Table 1. The EC50 (nM) and Emax (compared with NECA) values of Cl-IB-MECA in the calcium assay are 47 ± 9 nM and 57± 6%, respectively.
Similar articles
- Functionally biased modulation of A(3) adenosine receptor agonist efficacy and potency by imidazoquinolinamine allosteric enhancers.
Gao ZG, Verzijl D, Zweemer A, Ye K, Göblyös A, Ijzerman AP, Jacobson KA. Gao ZG, et al. Biochem Pharmacol. 2011 Sep 15;82(6):658-68. doi: 10.1016/j.bcp.2011.06.017. Epub 2011 Jun 21. Biochem Pharmacol. 2011. PMID: 21718691 Free PMC article. - Probing structure-activity relationship in β-arrestin2 recruitment of diversely substituted adenosine derivatives.
Storme J, Tosh DK, Gao ZG, Jacobson KA, Stove CP. Storme J, et al. Biochem Pharmacol. 2018 Dec;158:103-113. doi: 10.1016/j.bcp.2018.10.003. Epub 2018 Oct 4. Biochem Pharmacol. 2018. PMID: 30292756 Free PMC article. - Flexible modulation of agonist efficacy at the human A3 adenosine receptor by the imidazoquinoline allosteric enhancer LUF6000.
Gao ZG, Ye K, Göblyös A, Ijzerman AP, Jacobson KA. Gao ZG, et al. BMC Pharmacol. 2008 Dec 12;8:20. doi: 10.1186/1471-2210-8-20. BMC Pharmacol. 2008. PMID: 19077268 Free PMC article. - Partial agonists for A(3) adenosine receptors.
Gao ZG, Jacobson KA. Gao ZG, et al. Curr Top Med Chem. 2004;4(8):855-62. doi: 10.2174/1568026043450989. Curr Top Med Chem. 2004. PMID: 15078216 Free PMC article. Review. - Medicinal chemistry of the A3 adenosine receptor: agonists, antagonists, and receptor engineering.
Jacobson KA, Klutz AM, Tosh DK, Ivanov AA, Preti D, Baraldi PG. Jacobson KA, et al. Handb Exp Pharmacol. 2009;(193):123-59. doi: 10.1007/978-3-540-89615-9_5. Handb Exp Pharmacol. 2009. PMID: 19639281 Free PMC article. Review.
Cited by
- Nucleoside transporters and immunosuppressive adenosine signaling in the tumor microenvironment: Potential therapeutic opportunities.
Kaur T, Weadick B, Mace TA, Desai K, Odom H, Govindarajan R. Kaur T, et al. Pharmacol Ther. 2022 Dec;240:108300. doi: 10.1016/j.pharmthera.2022.108300. Epub 2022 Oct 22. Pharmacol Ther. 2022. PMID: 36283452 Free PMC article. Review. - Design, Synthesis and Evaluation of New Indolylpyrimidylpiperazines for Gastrointestinal Cancer Therapy.
Tan A, Babak MV, Venkatesan G, Lim C, Klotz KN, Herr DR, Cheong SL, Federico S, Spalluto G, Ong WY, Chen YZ, Loo JSE, Pastorin G. Tan A, et al. Molecules. 2019 Oct 11;24(20):3661. doi: 10.3390/molecules24203661. Molecules. 2019. PMID: 31614517 Free PMC article. - Assessment of biased agonism at the A3 adenosine receptor using β-arrestin and miniGαi recruitment assays.
Pottie E, Tosh DK, Gao ZG, Jacobson KA, Stove CP. Pottie E, et al. Biochem Pharmacol. 2020 Jul;177:113934. doi: 10.1016/j.bcp.2020.113934. Epub 2020 Mar 26. Biochem Pharmacol. 2020. PMID: 32224136 Free PMC article. - International Union of Basic and Clinical Pharmacology. CXII: Adenosine Receptors: A Further Update.
IJzerman AP, Jacobson KA, Müller CE, Cronstein BN, Cunha RA. IJzerman AP, et al. Pharmacol Rev. 2022 Apr;74(2):340-372. doi: 10.1124/pharmrev.121.000445. Pharmacol Rev. 2022. PMID: 35302044 Free PMC article. Review. - Therapeutic potential of β-arrestin- and G protein-biased agonists.
Whalen EJ, Rajagopal S, Lefkowitz RJ. Whalen EJ, et al. Trends Mol Med. 2011 Mar;17(3):126-39. doi: 10.1016/j.molmed.2010.11.004. Epub 2010 Dec 21. Trends Mol Med. 2011. PMID: 21183406 Free PMC article. Review.
References
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. - PubMed
- Madi L, Cohen S, Ochayin A, Bar-Yehuda S, Barer F, Fishman P. Overexpression of A3 adenosine receptor in peripheral blood mononuclear cells in rheumatoid arthritis: involvement of nuclear factor-kappaB in mediating receptor level. J Rheumatol. 2007;34:20–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous