Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors - PubMed (original) (raw)
Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors
Mounira Banasr et al. Biol Psychiatry. 2008.
Abstract
Background: Postmortem studies have repeatedly found decreased density and number of glia in cortical regions, including the prefrontal and cingulate areas, from depressed patients. However, it is unclear whether this glial loss plays a direct role in the expression of depressive symptoms.
Methods: To address this question, we characterized the effects of pharmacologic glial ablation in the prefrontal cortex (PFC) of adult rats on behavioral tests known to be affected by stress or antidepressant treatments: sucrose preference test (SPT), novelty suppressed feeding test (NSFT), forced swim test (FST), and two-way active avoidance test (AAT). We established the dose and time course for the actions of an astrocyte specific toxin, L-alpha-aminoadipic acid (L-AAA), and compared the behavioral effects of this gliotoxin with the effects of an excitotoxic (ibotenate) lesion and to the effects of chronic stress.
Results: The results demonstrate that L-AAA infusions induced anhedonia in SPT, anxiety in NSFT, and helplessness in FST and AAT. These effects of L-AAA were similar to chronic unpredictable stress (CUS)-induced depressive-like behaviors in these tests. However, ibotenate-induced neurotoxic lesion of the PFC had no effect in these behavioral tests.
Conclusions: The results demonstrate that glial ablation in the PFC is sufficient to induce depressive-like behaviors similar to chronic stress and support the hypothesis that loss of glia contributes to the core symptoms of depression.
Figures
Figure 1
Experimental design. Experiment 1, animals were subjected to the chronic unpredictable stress (CUS) procedure and tested in sucrose preference test (SPT), novelty suppressed feeding test (NSFT), forced swim test (FST) and active avoidance test (AAT). Experiment 4, animals were infused with saline, L-AAA or ibotenate and then subjected to the same 4 behavioral tests. Experiments 2 and 3 animals were infused with 50 and 100μg/μl of L-AAA into the PFC (Experiment 2) or with 100μg/μl of L-AAA or 5 μg/μl ibotenate (7 days after cannula implantation), and were then subjected to the SPT and FST immediately followed by a second SPT.
Figure 2
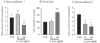
Effects of chronic unpredictable stress (CUS) on anhedonia, anxiety and helplessness. CUS and home cage control (HCC) animals were consecutively tested in sucrose preference (A, day 31), novelty suppressed feeding (B, day 32), forced swim (C, day 33) and active avoidance (D, day 34) tests. Results are expressed as mean ± s.e.m. ratio sucrose vs. water consumed (A), latency to feed in seconds (B), time spent immobile in seconds (C) and number of escape failures (D). * P<0.05, ** P<0.01, compared to CTR.
Figure 3
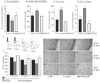
Effects of chronic unpredictable stress (CUS) on density of GFAP-positive cells in the prelimbic cortex (PL). Density of GFAP-positive cells was quantified in the PL of animals subjected to CUS and compared to home cage controls (HCC). Numbers of GFAP-positive cells were quantified throughout the PLC as shown in A (gray squares). (B) Results are expressed as mean ± s.e.m. of GFAP-positive cells/mm2. * P<0.05 compared to HCC. Representative GFAP stained sections from HCC (C) and CUS treated (D) animals are illustrated at approximately 2.7 mm from bregma. Scale bar = 200 μm
Figure 4
Effects of L-AAA infusions in the PFC on sucrose preference and forced swim tests. Two doses of L-AAA were tested (50 and 100μg/μl) and compared to saline infused animals (CTR). Rats were subjected to the sucrose preference test after 4h fluid deprivation (A, day 3), to forced swim test (B, day 5), and a second sucrose preference test (C, day 5). Results are expressed as mean ± s.e.m. ratio sucrose to water consumed (A and C) and time spent immobile in seconds (B). * P<0.05, ** P<0.01, compared to CTR.
Figure 5
Influence of L-AAA or ibotenate infusions in the PFC on behavior, astroglia and neurons. Animals received infusions of saline (CTR), L-AAA or ibotenate as indicated and were consecutively tested in sucrose preference (A, day 3), novelty suppressed feeding (B, day 4), forced swim (C, day 5) and active avoidance (D, day 6) tests. Results are expressed as mean ± s.e.m. ratio sucrose vs. water consumed (A), latency to feed in seconds (B), time spent immobile in seconds (C) and number of escape failures (D). * P<0.05, ** P<0.01, compared to CTR. Density of GFAP-positive cells was quantified in the PLC of animals subjected to L-AAA and ibotenate animals and compared to CTR. Number of GFAP-positive cells was quantified throughout the PLC as described in E (gray squares), and results are expressed as mean ± s.e.m. number of GFAP-positive cells/mm2 (F) * P<0.05, compared to CTR. Representative micrographs of GFAP (G–K) or NeuN immunostained sections (MO) showing the effects of L-AAA (H, K, N)) or ibotenate (I, L, O) are shown. The cannula site (4.2 mm from bregma) is marked by a star (*, G-I). Consequences of L-AAA or ibotenate on GFAP or NeuN immunostaining in fields away from the cannula site (level 2.7 mm from bregma) compared to saline infusions are shown (J–O). Scale bar = 100 μm.
Similar articles
- Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole.
Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, Sanacora G. Banasr M, et al. Mol Psychiatry. 2010 May;15(5):501-11. doi: 10.1038/mp.2008.106. Epub 2008 Sep 30. Mol Psychiatry. 2010. PMID: 18825147 Free PMC article. - Gap junction dysfunction in the prefrontal cortex induces depressive-like behaviors in rats.
Sun JD, Liu Y, Yuan YH, Li J, Chen NH. Sun JD, et al. Neuropsychopharmacology. 2012 Apr;37(5):1305-20. doi: 10.1038/npp.2011.319. Epub 2011 Dec 21. Neuropsychopharmacology. 2012. PMID: 22189291 Free PMC article. - Magnolol treatment reversed the glial pathology in an unpredictable chronic mild stress-induced rat model of depression.
Li LF, Yang J, Ma SP, Qu R. Li LF, et al. Eur J Pharmacol. 2013 Jul 5;711(1-3):42-9. doi: 10.1016/j.ejphar.2013.04.008. Epub 2013 Apr 28. Eur J Pharmacol. 2013. PMID: 23632393 - Antidepressant-like effect of the mGluR5 antagonist MTEP in an astroglial degeneration model of depression.
Domin H, Szewczyk B, Woźniak M, Wawrzak-Wleciał A, Śmiałowska M. Domin H, et al. Behav Brain Res. 2014 Oct 15;273:23-33. doi: 10.1016/j.bbr.2014.07.019. Epub 2014 Jul 18. Behav Brain Res. 2014. PMID: 25043733 - Antidepressant effects of fibroblast growth factor-2 in behavioral and cellular models of depression.
Elsayed M, Banasr M, Duric V, Fournier NM, Licznerski P, Duman RS. Elsayed M, et al. Biol Psychiatry. 2012 Aug 15;72(4):258-65. doi: 10.1016/j.biopsych.2012.03.003. Epub 2012 Apr 17. Biol Psychiatry. 2012. PMID: 22513055 Free PMC article.
Cited by
- Astrocytic P2X7 receptor regulates depressive-like behavioral reactions of mice in response to acute stressful stimulation.
Cheng XY, Ren WJ, Li X, Deussing JM, Illes P, Tang Y, Rubini P. Cheng XY, et al. Purinergic Signal. 2024 Sep 26. doi: 10.1007/s11302-024-10047-6. Online ahead of print. Purinergic Signal. 2024. PMID: 39325357 - Prefrontal cortex astrocytes in major depressive disorder: exploring pathogenic mechanisms and potential therapeutic targets.
Pan Y, Xiang L, Zhu T, Wang H, Xu Q, Liao F, He J, Wang Y. Pan Y, et al. J Mol Med (Berl). 2024 Nov;102(11):1355-1369. doi: 10.1007/s00109-024-02487-9. Epub 2024 Sep 14. J Mol Med (Berl). 2024. PMID: 39276178 - Antidepressant Effects of Ginsenoside Rc on L-Alpha-Aminoadipic Acid-Induced Astrocytic Ablation and Neuroinflammation in Mice.
Kwon D, Kim Y, Cho SH. Kwon D, et al. Int J Mol Sci. 2024 Sep 6;25(17):9673. doi: 10.3390/ijms25179673. Int J Mol Sci. 2024. PMID: 39273621 Free PMC article. - Astrocyte-derived dominance winning reverses chronic stress-induced depressive behaviors.
Noh K, Oh J, Cho WH, Hwang M, Lee SJ. Noh K, et al. Mol Brain. 2024 Aug 27;17(1):59. doi: 10.1186/s13041-024-01134-1. Mol Brain. 2024. PMID: 39192323 Free PMC article. - Astrocytes in the Ventral Hippocampus Bidirectionally Regulate Innate and Stress-Induced Anxiety-Like Behaviors in Male Mice.
Li JT, Jin SY, Hu J, Xu RX, Xu JN, Li ZM, Wang ML, Fu YW, Liao SH, Li XW, Chen YH, Gao TM, Yang JM. Li JT, et al. Adv Sci (Weinh). 2024 Oct;11(38):e2400354. doi: 10.1002/advs.202400354. Epub 2024 Aug 9. Adv Sci (Weinh). 2024. PMID: 39120568 Free PMC article.
References
- Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, et al. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51:273–279. - PubMed
- Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res. 2000;126:413–431. - PubMed
- Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry. 2003;54:338–352. - PubMed
- Banasr M, Duman RS. Regulation of neurogenesis and gliogenesis by stress and antidepressant treatment. CNS Neurol Disord Drug Targets. 2007;6:311–320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 MH025642-300028/MH/NIMH NIH HHS/United States
- P01 MH025642/MH/NIMH NIH HHS/United States
- 2 P01 MH25642/MH/NIMH NIH HHS/United States
- R37 MH045481-19/MH/NIMH NIH HHS/United States
- R37 MH045481-16/MH/NIMH NIH HHS/United States
- MH45481/MH/NIMH NIH HHS/United States
- R37 MH045481/MH/NIMH NIH HHS/United States
- R37 MH045481-17/MH/NIMH NIH HHS/United States
- P01 MH025642-310028/MH/NIMH NIH HHS/United States
- P01 MH025642-320028/MH/NIMH NIH HHS/United States
- R01 MH045481/MH/NIMH NIH HHS/United States
- P01 MH025642-31/MH/NIMH NIH HHS/United States
- P01 MH025642-32/MH/NIMH NIH HHS/United States
- R37 MH045481-18/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous