Noncanonical DNA motifs as transactivation targets by wild type and mutant p53 - PubMed (original) (raw)
Noncanonical DNA motifs as transactivation targets by wild type and mutant p53
Jennifer J Jordan et al. PLoS Genet. 2008.
Erratum in
- PLoS Genet. 2008 Jul;4(7). doi: 10.1371/annotation/13bc83be-2345-401d-b953-f1886e9fbdff. Nourredine, Maher [corrected to Noureddine, Maher]; Bell, Douglas [corrected to Bell, Douglas A]
- PLoS Genet. 2008 Nov;4(11).doi.org/10.1371/annotation/f7fc9c28-14ae-480d-a69e-ee9cc4fba9a7
Abstract
Sequence-specific binding by the human p53 master regulator is critical to its tumor suppressor activity in response to environmental stresses. p53 binds as a tetramer to two decameric half-sites separated by 0-13 nucleotides (nt), originally defined by the consensus RRRCWWGYYY (n = 0-13) RRRCWWGYYY. To better understand the role of sequence, organization, and level of p53 on transactivation at target response elements (REs) by wild type (WT) and mutant p53, we deconstructed the functional p53 canonical consensus sequence using budding yeast and human cell systems. Contrary to early reports on binding in vitro, small increases in distance between decamer half-sites greatly reduces p53 transactivation, as demonstrated for the natural TIGER RE. This was confirmed with human cell extracts using a newly developed, semi-in vitro microsphere binding assay. These results contrast with the synergistic increase in transactivation from a pair of weak, full-site REs in the MDM2 promoter that are separated by an evolutionary conserved 17 bp spacer. Surprisingly, there can be substantial transactivation at noncanonical (1/2)-(a single decamer) and (3/4)-sites, some of which were originally classified as biologically relevant canonical consensus sequences including PIDD and Apaf-1. p53 family members p63 and p73 yielded similar results. Efficient transactivation from noncanonical elements requires tetrameric p53, and the presence of the carboxy terminal, non-specific DNA binding domain enhanced transactivation from noncanonical sequences. Our findings demonstrate that RE sequence, organization, and level of p53 can strongly impact p53-mediated transactivation, thereby changing the view of what constitutes a functional p53 target. Importantly, inclusion of (1/2)- and (3/4)-site REs greatly expands the p53 master regulatory network.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
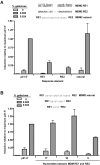
Figure 1. Isogenomic diploid yeast system to investigate transcriptional capacity of p53 towards many REs at various p53 levels.
(A) Transactivation capacities of p53 (WT or mutant) from cognate REs were determined using diploid strains derived from haploids where p53 and reporters with upstream REs were integrated into different chromosomal loci. Within the p53-host strains, p53 (WT or mutant) was controlled by a “rheostatable” GAL1 promoter (blue cells) (previously described [109]) which allows for controlled, inducible expression of p53. Reporter strains contained promoter REs upstream of the minimal CYC1 promoter and either the ADE2 color reporter (REP) or the firefly luciferase reporter (tan cells) . Mating of the REP and p53-host strains results in isogenomic diploid yeast that provide for rapid assessment of the transactivation potential for WT or mutant p53 proteins towards many individual REs in the p53 transcriptional network . (B) Inducible expression of p53 under the rheostatable GAL1 promoter. The GAL1 promoter allows for controlled expression of p53 depending on the level of galactose in the media. Raffinose was added to provide a basal level of expression, as the GAL1 promoter is derepressed, but not induced in media containing this carbon source. Presented is a Western blot analysis of p53 expression 24 hours post-inoculation with raffinose or raffinose plus increasing amounts of galactose (0.004–0.032%). Increases in galactose from 0–0.032% correlated with an increase in p53 over a 100-fold range. The p53 protein was detected with DO-1 and pAb1801 antibodies. The asterisk (*) depicts a longer exposure to reveal protein at basal and lower galactose levels. GAPDH, identified by immunodetection provided a standard loading control. (C) Quantitative assessment of p53-induced transactivation from the p21-5′ RE in vivo. The diploid yeast strain containing GAL1::WT p53 crossed with the p21-5′ RE-luciferase REP was grown overnight in complete medium, diluted, washed and inoculated into selective medium containing either raffinose or raffinose plus increasing galactose (0–0.032%) for 24 hours. Protein lysates were obtained and a luciferase assay was used to determine the transactivation capacity of p53 from the p21-5′ RE. The strength of transactivation was calculated as relative light units/µg protein. Circled are the basal, linear-increase, and plateau phases of the transactivation response as a function of galactose concentration and are referred to as basal, moderate and high levels of p53 expression.
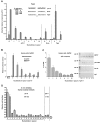
Figure 2. Weak REs can function synergistically when separated by a spacer.
(A) To ascertain if p53 functions from the two full-site REs of MDM2 independently or if the REs interact, p53 transactivation from the isolated REs, as well as the natural MDM2 RE containing a 17 nucleotide spacer were evaluated. Isogenomic diploid yeast strains containing the p21-5′ and MDM2 REs, as indicated, were grown in increasing concentrations of galactose to induce p53 protein to basal, moderate and high levels of expression. The ability of WT p53 to transactivate from RE sequences was measured by a luciferase assay 24 hours after inoculation into the galactose supplemented media. Induction from each RE was compared relative to the ability of p53 to transactivate from the p21-5′ RE at 0.024% galactose and is depicted as the mean and standard error of measurement (SEM) of 6 independent experiments. The average light units/µg protein from p21-5′ at 0.024% galactose was 2.1 million. Solid arrows over the sequences indicate a ¼-binding site. (B) Impact of reducing the spacer to 10 and 5 nucleotides. The average light units/µg protein for p21-5′ at 0.024% galactose was 2,800,000.
Figure 3. Spacer decreases p53 transactivation, promoter occupancy and binding in yeast and mammalian cells and in vitro.
(A) Transactivation in yeast. The ability of p53 to transactivate from REs containing spacers of variable nucleotide length sequences was measured 24 hours after p53 induction with a quantitative luciferase assay. Induction from each RE at various p53 expression levels were compared to the induction from the p21-5′ RE at 0.024% galactose. The average light units/µg protein for WT p53 towards p21-5′ at 0.024% galactose for a minimum of 6 biological repeats was 1.26 million. *indicates the number of nucleotides in the spacer of the natural RE. Solid arrows identify a ¼-binding site. (B) Human SaOS2 cells were transfected with p21-5′::luciferase reporter constructs containing spacers of increasing length between decamer half-sites in the presence (solid bars) or absence (open bars) of the high expressing pCMV-p53 WT vector. At 48 hours post transfection, induction of the luciferase reporter was assessed. Relative luciferase activity was compared to the pGL3-P plasmid lacking the p53 RE (mock) and is represented by the average and standard deviations of three independent experiments, each containing three replicates. (C) Occupancy of p53 at p21-5′ REs in human cells. The p21-5′ promoter constructs containing the increasing spacers between half-sites were co-transfected with p53 into SaOS2 cells (as described in (B)). Twenty-four hours later, ChIP analysis was performed. Presented are the average and standard deviation from 4 independent experiments (left). PCR products of the Input DNA (input) and ChIP DNA (p53, IgG, or no antibody, Ab) are shown (right). The “M” corresponds to a pGL3-P plasmid control lacking the p53 RE. No bands were observed above the 600 bp markers. (D) In vitro fluorescent microsphere binding assay to evaluate sequence-specific p53-DNA binding interactions (see Materials and Methods). Fluorescent microspheres bearing double stranded DNA fragments were multiplexed and incubated in the presence of nuclear extracts from non-treated (NT) or Doxo-treated (0.6 ug/mL [1 mM] Doxorubicin for 18 hours at 37°C) lymphoblastoid cells. The DNA fragments contained the p21-5′ RE, p21-5′ RE with spacers of increasing length (0–15), p21-5′ half-site RE (left or right), or a scramble sequence.
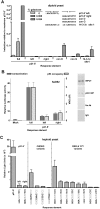
Figure 4. Half-sites function as noncanonical REs for transactivation in a sequence-dependent manner in yeast and human cells.
(A) Transactivation from decamer REs in the diploid yeast was quantified with a luciferase assay. Protein lysates were obtained 24 hours post inoculation into galactose supplemented media. The average light units/µg protein for WT p53 towards p21-5′ at 0.024% galactose for six biological repeats was 1.8 million. Solid arrows indicate ¼-binding sites. Comparisons were made with transactivation from the p21-5′ full site at high protein levels. (B) Transactivation in human SaOS2 cells. The cells were co-transfected with WT p53 along with either the full, left or right half-sites of the p21-5′ RE containing reporter construct. Transactivation was assessed with the luciferase assay 48 hours later. Relative luciferase activity was compared to a mock transfection containing the promoter-less pGL3 plasmid. Presented are the averages and standard deviations of 3 independent experiments that were each done in triplicate. PCR products of the input DNA (input) and ChIP DNA (p53, IgG or no antibody, Ab) are shown (right). Input and ChIP PCR products for the mock and p21-5′ full-site are shown in Figure 3C. No bands were observed above the 600 bp markers. (C) Sequence dependence of p53 transactivation from decamer half-sites. The extent to which p53 transactivation from half-sites is sequence dependent at high expression levels (2% galactose) was determined with a plasmid-based haploid yeast system . Relative light units/µg protein from ½-sites were compared to transactivation from the yLFM strain containing the p21-5′ full-site RE. The average light units/µg protein from p21-5′ at 2% galactose was 2.9 million.
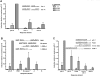
Figure 5. A ¾-site can function as a noncanonical RE in p53 transactivation.
(A) Transactivation was assessed from ¾ REs Con J and Con K. The ability of WT p53 to transactivate was measured with the diploid yeast luciferase assay 24 hours post inoculation into the galactose-supplemented media. Induction from each RE was compared relative to the ability of p53 to transactivate from the p21-5′ RE at 0.024% galactose and is depicted as the mean and SEM of 6 independent experiments. The average light units/µg protein from p21-5′ at 0.024% galactose was 2.1 million. Solid arrows indicate a ¼-binding site. (B) p53 functions from a ¾-site RE in the PIDD RE. Removal of the natural 8 bp spacer increased transactivation as expected; however, p53 was also able to transactivate from the natural PIDD RE containing the spacer. To determine the true functional p53 binding element, the canonical PIDD RE was broken into two noncanonical sites, ¾ PIDD-A and ¾ PIDD-B, which incorporated the spacer sequence into the RE and assessed for transactivation capacity with the luciferase assay. Solid arrows indicate ¼-binding site; dashed arrows indicate putative ¼-binding sites. (C) Analysis of p53 transactivation from various p53 targets in the genome that may be functioning ¾-sites. Shown are the identified p53 target sequences for p21-3′, PCNA, 14-3-3σ site 2 and APAF1 with the noncanonical ¾-site RE contained in these sites (identified by solid arrows).
Similar articles
- Estrogen receptor acting in cis enhances WT and mutant p53 transactivation at canonical and noncanonical p53 target sequences.
Menendez D, Inga A, Resnick MA. Menendez D, et al. Proc Natl Acad Sci U S A. 2010 Jan 26;107(4):1500-5. doi: 10.1073/pnas.0909129107. Epub 2010 Jan 4. Proc Natl Acad Sci U S A. 2010. PMID: 20080630 Free PMC article. - Potentiating the p53 network.
Menendez D, Inga A, Resnick MA. Menendez D, et al. Discov Med. 2010 Jul;10(50):94-100. Discov Med. 2010. PMID: 20670604 Review. - Differential transactivation by the p53 transcription factor is highly dependent on p53 level and promoter target sequence.
Inga A, Storici F, Darden TA, Resnick MA. Inga A, et al. Mol Cell Biol. 2002 Dec;22(24):8612-25. doi: 10.1128/MCB.22.24.8612-8625.2002. Mol Cell Biol. 2002. PMID: 12446780 Free PMC article. - Discrimination of DNA binding sites by mutant p53 proteins.
Thukral SK, Lu Y, Blain GC, Harvey TS, Jacobsen VL. Thukral SK, et al. Mol Cell Biol. 1995 Sep;15(9):5196-202. doi: 10.1128/MCB.15.9.5196. Mol Cell Biol. 1995. PMID: 7651437 Free PMC article. - Roles of p53 Family Structure and Function in Non-Canonical Response Element Binding and Activation.
Cai BH, Chao CF, Huang HC, Lee HY, Kannagi R, Chen JY. Cai BH, et al. Int J Mol Sci. 2019 Jul 27;20(15):3681. doi: 10.3390/ijms20153681. Int J Mol Sci. 2019. PMID: 31357595 Free PMC article. Review.
Cited by
- Structure of p73 DNA-binding domain tetramer modulates p73 transactivation.
Ethayathulla AS, Tse PW, Monti P, Nguyen S, Inga A, Fronza G, Viadiu H. Ethayathulla AS, et al. Proc Natl Acad Sci U S A. 2012 Apr 17;109(16):6066-71. doi: 10.1073/pnas.1115463109. Epub 2012 Apr 2. Proc Natl Acad Sci U S A. 2012. PMID: 22474346 Free PMC article. - Distinct p53 genomic binding patterns in normal and cancer-derived human cells.
Botcheva K, McCorkle SR, McCombie WR, Dunn JJ, Anderson CW. Botcheva K, et al. Cell Cycle. 2011 Dec 15;10(24):4237-49. doi: 10.4161/cc.10.24.18383. Epub 2011 Dec 15. Cell Cycle. 2011. PMID: 22127205 Free PMC article. - Interferon regulatory factor 4 binding protein is a novel p53 target gene and suppresses cisplatin-induced apoptosis of breast cancer cells.
Yang M, Yuan F, Li P, Chen Z, Chen A, Li S, Hu C. Yang M, et al. Mol Cancer. 2012 Aug 13;11:54. doi: 10.1186/1476-4598-11-54. Mol Cancer. 2012. PMID: 22888789 Free PMC article. - More targets, more pathways and more clues for mutant p53.
Garritano S, Inga A, Gemignani F, Landi S. Garritano S, et al. Oncogenesis. 2013 Jul 1;2(7):e54. doi: 10.1038/oncsis.2013.15. Oncogenesis. 2013. PMID: 23817466 Free PMC article. - Formation of stress-specific p53 binding patterns is influenced by chromatin but not by modulation of p53 binding affinity to response elements.
Millau JF, Bandele OJ, Perron J, Bastien N, Bouchard EF, Gaudreau L, Bell DA, Drouin R. Millau JF, et al. Nucleic Acids Res. 2011 Apr;39(8):3053-63. doi: 10.1093/nar/gkq1209. Epub 2010 Dec 21. Nucleic Acids Res. 2011. PMID: 21177650 Free PMC article.
References
- Ko LJ, Prives C. p53: puzzle and paradigm. Genes Dev. 1996;10:1054–1072. - PubMed
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. - PubMed
- Menendez D, Inga A, Jordan JJ, Resnick MA. Changing the p53 master regulatory network: ELEMENTary, my dear Mr Watson. Oncogene. 2007;26:2191–2201. - PubMed
- Raver-Shapira N, Marciano E, Meiri E, Spector Y, Rosenfeld N, et al. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell. 2007;26:731–743. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous