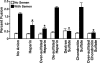The cationic properties of SEVI underlie its ability to enhance human immunodeficiency virus infection - PubMed (original) (raw)
The cationic properties of SEVI underlie its ability to enhance human immunodeficiency virus infection
Nadia R Roan et al. J Virol. 2009 Jan.
Abstract
Human semen contains peptides capable of forming amyloid fibrils termed semen-derived enhancer of viral infection (SEVI) that can greatly increase human immunodeficiency virus (HIV) infection. While SEVI appears to enhance virion attachment to target cells, its underlying mechanism of action is unknown. We now demonstrate that the intrinsic positive charges of SEVI (pI = 10.21) facilitate virion attachment to and fusion with target cells. A mutant form of SEVI in which lysines and arginines are replaced with alanines retains the ability to form amyloid fibrils but is defective in binding virions and enhancing infection. In addition, the interaction of wild-type SEVI with virions and the ability of these fibrils to increase infection are abrogated in the presence of various polyanionic compounds. These anionic polymers also decrease the enhancement of HIV infection mediated by semen. These findings suggest that SEVI enhances viral infection by serving as a polycationic bridge that neutralizes the negative charge repulsion that exists between HIV virions and target cells. Combinations of agents that neutrale SEVI action and produce HIV virucidal effects are an attractive future direction for microbicide development.
Figures
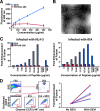
FIG. 1.
PAP248-286 forms fibrils and enhances the fusion of HIV-1 to primary CD4+ T cells. (A) Increasing concentrations of agitated PAP248-286 or PAP248-266 were mixed with 5 μM thioflavin T, and emission at 482 nm was recorded. Results are representative of data obtained from three independent experiments with two different batches of synthetic peptide. Shown are average values (± standard deviations) of triplicate measurements. (B) Electron micrograph of PAP248-286 fibrils (500 μg/ml). White bar = 200 nm. (C) CD14− CD4+ T cells were infected with BlaM-Vpr-containing NL4-3 or 81A virions in the presence of increasing concentrations of either SEVI or agitated PAP248-266 and then analyzed for virion fusion. The entry inhibitors AMD3100 and TAK779 were added to the indicated samples. Results are reported as _n_-fold enhancement of fusion relative to the level of fusion in the absence of any added peptide. These results were confirmed in two independent experiments. (D) CD3+ CD4+ T cells isolated from endometrial biopsy samples were infected with BlaM-Vpr-containing 81A virions in the absence or presence of SEVI. Bottom left: fluorescence-activated cell sorter plots for the detection of virion fusion from a single donor. Values at the bottom right of plots reflect the percentages of cells that fused with virions. Right: _n_-fold enhancement of fusion in the presence of SEVI (n = 5 donors). Blue lines are from experiments with CD4+ T cells purified directly from endometrial tissues. Red lines are from experiments with CD4+ T cells purified from endometrial “crawl-out” cells. FSC, forward scatter; SSC, side scatter.
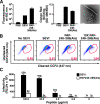
FIG. 2.
PAP248-286(Ala) forms fibrils but is deficient in enhancing HIV infection. (A) Solutions containing 250 μg/ml agitated PAP248-286 (SEVI) and PAP248-286(Ala) were monitored by thioflavin T fluorescence (left panel) or Congo red binding (center panel). The right panel shows an electron micrograph of PAP248-286(Ala) fibrils (500 μg/ml). White bar = 200 nm. (B) CD14− CD4+ lymphocytes were infected with BlaM-Vpr-containing NL4-3 in the presence of SEVI, agitated PAP248-286(Ala), or 10-fold more agitated PAP248-286(Ala) than SEVI. Values at the bottom right of the fluorescence-activated cell sorter plots reflect the percentages of cells that fused with virions. Results are gated on CD3+ CD4+ cells. The experiment was repeated four times with similar results. (C) TZM-bl cells were infected with CCR5-tropic HIV virions in the presence of SEVI or agitated PAP248-286(Ala). Cells were assayed for β-galactosidase activity 3 days postinfection. The values indicate the _n_-fold infectivity enhancement relative to the infectivity in the absence of peptide. Results are representative of data from three separate experiments. OD, optical density; RLU, relative light units.
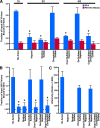
FIG. 3.
SEVI fibrils, unlike PAP248-286(Ala) fibrils, efficiently bind HIV virions in a manner that can be blocked by some anionic polymers. (A) SEVI or PAP248-286(Ala) fibrils were incubated with NL4-3 virions in the presence of the indicated anionic polymers and centrifuged. The absolute amounts of p24Gag in the pellet and supernatant were determined by ELISA. Values are the mean ± standard deviation from one of four experiments that yielded similar results. *, P < 0.01 versus SEVI in the absence of anionic compound (two-tailed t test). LOD, limit of detection determined by the p24Gag signal in the absence of peptide. (B) SEVI was pretreated with the indicated anionic polymers and then centrifuged to pellet fibrils and any interacting polymers. Pellets were then incubated with HIV, and the proportion of virions bound to fibrils was determined as for panel A. Values are the mean ± standard deviation of three experiments. *, P < 0.01 versus SEVI in the absence of anion (two-tailed t test). LOD, limit of detection determined by the p24Gag signal in the absence of peptide. (C) TZM-bl cells were incubated with virion-exposed SEVI fibrils that were treated as described in Fig. 3B. β-Galactosidase activity was measured 3 days postinfection.
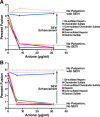
FIG. 4.
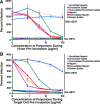
Anionic polymers inhibit SEVI-mediated enhancement of viral fusion. (A) BlaM-Vpr-containing NL4-3 virions were incubated with SEVI and different concentrations of the indicated polyanionic compounds. The virions were then diluted fivefold to a final concentration of 50 ng/ml and added to CD14− CD4+ T cells. The level of viral fusion in the absence of SEVI is indicated by the bottom dotted line, whereas the level of viral fusion in the presence of SEVI is indicated by the top dotted line. At concentrations greater than 8 μg/ml, heparin, oversulfated heparin, dextran sulfate, and oversulfated chondroitin sulfate inhibited SEVI activity. Desulfated heparin and chondroitin sulfate had minimal inhibitory effect at all of the concentrations tested. Results are representative of data from five separate experiments. (B) CD14− CD4+ T cells were incubated with SEVI and different concentrations of the indicated anionic polymers. The cells were then washed and infected with BlaM-Vpr-containing NL4-3 and analyzed by flow cytometry for virion fusion. The level of viral fusion in the absence of SEVI is indicated by the bottom line, whereas the level of viral fusion in the presence of SEVI is indicated by the top dotted line. At concentrations greater than 8 μg/ml, heparin, oversulfated heparin, dextran sulfate, and oversulfated chondroitin sulfate inhibited SEVI activity. Desulfated heparin and chondroitin sulfate had minimal inhibitory effect at all of the concentrations tested. Results are representative of data from five separate experiments.
FIG. 5.
Anionic polymer treatment of HIV and T cells in the absence of SEVI. (A) BlaM-Vpr-containing NL4-3 virions treated with the indicated polyanionic compounds were added to CD14− CD4+ T cells. Values at the bottom right of plots reflect the percentages of cells that fused with virions. Treatment of virions with dextran sulfate and oversulfated heparin essentially eliminated viral fusion. (B) CD14− CD4+ T cells were treated with the indicated anionic polymers, washed to remove excess polymers, and infected with BlaM-Vpr-containing NL4-3 virions. Values at the bottom right of plots reflect the percentages of cells that fused with virions. Importantly, none of the polyanionic compound-treated cells were completely unable to fuse with virions.
FIG. 6.
Polyanionic compounds inhibit SEVI-mediated enhancement of HIV infection. (A) Virions were pretreated with SEVI and the indicated polyanionic compounds and then added to TZM-bl cells. (B) Alternatively, TZM-bl cells pretreated with the indicated anionic polymers were infected with HIV in the presence of SEVI. Cells were assayed for luciferase activity 2 days postinfection. Samples containing SEVI are graphed with circles, while samples lacking SEVI are graphed with squares. The polyanionic compounds that were used for the SEVI-treated samples are labeled. Samples treated with polyanions in the absence of SEVI are not labeled; however, the colors for these samples correspond to those for the samples containing SEVI. The values on the y axis were calculated based on the percent infection relative to samples infected in the absence of SEVI and anionic polymers. Shown are data representative of three independent experiments.
FIG. 7.
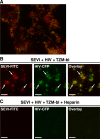
Anionic polymers inhibit binding of SEVI to host cells. HEK293 supernatants containing CFP-labeled HIV virions were preincubated with FITC-conjugated SEVI in the absence or presence of heparin and then added to TZM-bl cells. Samples were then fixed and visualized by fluorescence microscopy. SEVI-FITC is shown in red, and HIV-CFP is shown in green. (A) High-resolution merged image demonstrating the binding of HIV virions to SEVI. (B) Binding of HIV virions to SEVI in the absence of heparin. Arrows indicate examples of SEVI/HIV complexes. (C) Heparin abolishes binding of SEVI and HIV virions to cells. Bars = 10 μm.
FIG. 8.
Anionic polymers inhibit semen-mediated enhancement of viral fusion. CD14− CD4+ T cells were incubated with medium or 10% semen in the presence of polyanionic compounds. The cells were then washed and infected with BlaM-Vpr-containing NL4-3, and the level of virion fusion was determined by flow cytometry. Values are the mean ± standard deviation from one of four experiments that yielded similar results. *, P < 0.01 versus with semen in the absence of anion (one-tailed t test).
FIG. 9.
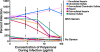
Polyanionic compounds inhibit semen-mediated enhancement of HIV infection. Virions were treated with 10% diluted semen or mock treated for 10 min and then added to TZM-bl cells in the presence of the indicated anionic polymer. After 2 h of incubation at 37°C, the supernatant was removed and the cells were further cultivated in fresh medium. Cells were assayed for β-galactosidase activity 2 days postinfection. Samples containing semen are graphed with circles, while samples lacking semen are graphed with squares. Labeling of the graph is similar to the labeling used in Fig. 6. The values on the y axis were calculated based on the percent infection relative to samples infected in the absence of semen and polyanionic compounds. Similar results were obtained in two independent experiments.
Similar articles
- Myricetin antagonizes semen-derived enhancer of viral infection (SEVI) formation and influences its infection-enhancing activity.
Ren R, Yin S, Lai B, Ma L, Wen J, Zhang X, Lai F, Liu S, Li L. Ren R, et al. Retrovirology. 2018 Jul 16;15(1):49. doi: 10.1186/s12977-018-0432-3. Retrovirology. 2018. PMID: 30012153 Free PMC article. - Aminoquinoline surfen inhibits the action of SEVI (semen-derived enhancer of viral infection).
Roan NR, Sowinski S, Münch J, Kirchhoff F, Greene WC. Roan NR, et al. J Biol Chem. 2010 Jan 15;285(3):1861-9. doi: 10.1074/jbc.M109.066167. Epub 2009 Nov 6. J Biol Chem. 2010. PMID: 19897482 Free PMC article. - Characterization of the Influence of Semen-Derived Enhancer of Virus Infection on the Interaction of HIV-1 with Female Reproductive Tract Tissues.
Allen SA, Carias AM, Anderson MR, Okocha EA, Benning L, McRaven MD, Kelley ZL, Lurain J, Veazey RS, Hope TJ. Allen SA, et al. J Virol. 2015 May;89(10):5569-80. doi: 10.1128/JVI.00309-15. Epub 2015 Mar 4. J Virol. 2015. PMID: 25740984 Free PMC article. - [Semen-derived enhancer of viral infection--a key factor in sexual transmission of HIV].
Duan JM, Qiu JY, Tan SY, Liu SW, Li L. Duan JM, et al. Bing Du Xue Bao. 2012 Jan;28(1):84-8. Bing Du Xue Bao. 2012. PMID: 22416356 Review. Chinese. - Natural Seminal Amyloids as Targets for Development of Synthetic Inhibitors of HIV Transmission.
Sheik DA, Dewhurst S, Yang J. Sheik DA, et al. Acc Chem Res. 2017 Sep 19;50(9):2159-2166. doi: 10.1021/acs.accounts.7b00154. Epub 2017 Aug 15. Acc Chem Res. 2017. PMID: 28809479 Review.
Cited by
- Bacterial curli protein promotes the conversion of PAP248-286 into the amyloid SEVI: cross-seeding of dissimilar amyloid sequences.
Hartman K, Brender JR, Monde K, Ono A, Evans ML, Popovych N, Chapman MR, Ramamoorthy A. Hartman K, et al. PeerJ. 2013 Feb 12;1:e5. doi: 10.7717/peerj.5. Print 2013. PeerJ. 2013. PMID: 23638387 Free PMC article. - Sexual Transmission of XMRV: A Potential Infection Route.
Sharma P, Rogers KA, Suppiah S, Molinaro RJ, Onlamoon N, Hackett J Jr, Schochetman G, Klein EA, Silverman RH, Villinger F. Sharma P, et al. Adv Virol. 2011;2011:965689. doi: 10.1155/2011/965689. Epub 2011 Jul 24. Adv Virol. 2011. PMID: 22312360 Free PMC article. - [PSB0739 inhibits formation of semen-derived amyloid fibril].
Lan Y, Yang Z, Liu H, Cheng H, Liu S, Tan S. Lan Y, et al. Nan Fang Yi Ke Da Xue Xue Bao. 2018 Nov 30;38(11):1338-1343. doi: 10.12122/j.issn.1673-4254.2018.11.10. Nan Fang Yi Ke Da Xue Xue Bao. 2018. PMID: 30514682 Free PMC article. Chinese. - The anti-parasitic drug suramin potently inhibits formation of seminal amyloid fibrils and their interaction with HIV-1.
Tan S, Li JQ, Cheng H, Li Z, Lan Y, Zhang TT, Yang ZC, Li W, Qi T, Qiu YR, Chen Z, Li L, Liu SW. Tan S, et al. J Biol Chem. 2019 Sep 13;294(37):13740-13754. doi: 10.1074/jbc.RA118.006797. Epub 2019 Jul 25. J Biol Chem. 2019. PMID: 31346035 Free PMC article. - Structure-based design of non-natural amino-acid inhibitors of amyloid fibril formation.
Sievers SA, Karanicolas J, Chang HW, Zhao A, Jiang L, Zirafi O, Stevens JT, Münch J, Baker D, Eisenberg D. Sievers SA, et al. Nature. 2011 Jun 15;475(7354):96-100. doi: 10.1038/nature10154. Nature. 2011. PMID: 21677644 Free PMC article.
References
- Appay, V., and S. L. Rowland-Jones. 2001. RANTES: a versatile and controversial chemokine. Trends Immunol. 2283-87. - PubMed
- Bouvet, J. P., G. Gresenguet, and L. Belec. 1997. Vaginal pH neutralization by semen as a cofactor of HIV transmission. Clin. Microbiol. Infect. 319-23. - PubMed
- Cavrois, M., C. De Noronha, and W. C. Greene. 2002. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat. Biotechnol. 201151-1154. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous