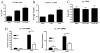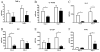Critical role of IL-17RA in immunopathology of influenza infection - PubMed (original) (raw)
Critical role of IL-17RA in immunopathology of influenza infection
Christopher R Crowe et al. J Immunol. 2009.
Abstract
Acute lung injury due to influenza infection is associated with high mortality, an increase in neutrophils in the airspace, and increases in tissue myeloperoxidase (MPO). Because IL-17A and IL-17F, ligands for IL-17 receptor antagonist (IL-17RA), have been shown to mediate neutrophil migration into the lung in response to LPS or Gram-negative bacterial pneumonia, we hypothesized that IL-17RA signaling was critical for acute lung injury in response to pulmonary influenza infection. IL-17RA was critical for weight loss and both neutrophil migration and increases in tissue myeloperoxidase (MPO) after influenza infection. However, IL-17RA was dispensable for the recruitment of CD8(+) T cells specific for influenza hemagglutinin or nucleocapsid protein. Consistent with this, IL-17RA was not required for viral clearance. However, in the setting of influenza infection, IL-17RA(-/-) mice showed significantly reduced levels of oxidized phospholipids, which have previously been shown to be an important mediator in several models of acute lung injury, including influenza infection and gastric acid aspiration. Taken together, these data support targeting IL-17 or IL-17RA in acute lung injury due to acute viral infection.
Conflict of interest statement
Disclosures
The authors have no financial conflict of interest.
Figures
FIGURE 1
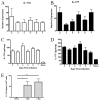
IL-17A and IL-17F are expressed in response to influenza. Time course, in BALB/cJ mice, of IL-17A (A and C) and IL-17F (B and D) following influenza challenge. mRNA was detected by quantitative real-time PCR and is expressed relative to naive levels, with all time points having a p value of <0.05 compared with naive controls. Protein levels were detected by ELISA, and all time points had a p value of <0.05 compared with naive controls. E, The number of IL-17A-producing cells was quantified by ELISpot. *, p = 0.016; **, p = 0.003, n = 4/time point.
FIGURE 2
Production of IL-17 by γδ T cells. Intracellular cytokine staining following influenza challenge demonstrates an increasing percentage of IL-17+ γδ T cells (A), even as the total population of γδ T cells expands (B) Percentage of IL-17+ αβ T cells is unchanged compared with naive mice (C) Challenge of γδ knockout mice results in lower levels of IL-17A and IL-17F message, expressed as fold change from naive, at 6 dpi (D). *, p = 0.03; **, p = 0.02, n = 8/time point.
FIGURE 3
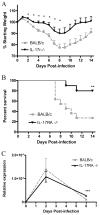
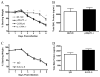
Weight loss and mortality following challenge with influenza A/PR/8. Weight loss (A) and mortality (B) were assessed daily following viral challenge (n = 10/group). Viral burden (C) was determined by quantitative PCR for the influenza M1 protein (n = 4/group/time point). *, p < 0.05; **, p = 0.0067; ***, p = 0.0093.
FIGURE 4
Differences in inflammation following influenza challenge. BALB/c (A) and IL-17RA−/− (B) lungs at 6 dpi, inflated with 10% formalin, paraffin-embedded, and H&E stained. Calibration bar, 100 _μ_m. Pathology scored (C) as in Lung fixation and histological examination. *, p = 0.01.
FIGURE 5
Cytokine response to influenza challenge. Cytokines measured at 2 and 6 dpi. BALB/c (□) vs IL-17RA−/− (■). *, p < 0.05 (n = 4/group/time point; A).
FIGURE 6
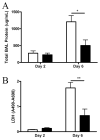
Markers of lung injury. Total protein (A) and LDH activity (B) in the BALF. □, BALB/c mice; ■, IL-17RA−/− mice. *, p = 0.01; **, p = 0.01 (n = 4/group/time point).
FIGURE 7
Protection is not dependent on TNF-α or IL-6. A, Weight loss among B6.129 and p55/p75 (n = 6/group) following influenza challenge. For comparison, IL-17RA−/− on a C57BL/6 background and wild-type controls (n = 4/group) are also provided. B, Lung injury assessed by total protein in the BALF on day 6. C, BALB/c mice were treated with either anti-IL-6 (n = 4) or control IgG (n = 4) before challenge and again on 3 dpi, weight loss monitored for 6 days and lavaged on day 6 (D) to measure total protein in the BALF. *, p < 0.05.
FIGURE 8
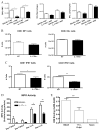
Differences in neutrophils and oxidized phospholipids. A, IL-17RA−/− mice (■) show decreases in the number of neutrophils in the BAL compared with BALB/c controls (□), but no differences in macrophages or lymphocytes (n = 4/group/time point). B, Pentamer staining at 10 dpi show that there is no difference in the numbers of HA-specific or NP-specific CD8+ T cells (n = 8/group). C, The numbers of IFN-γ positive CD4+ and CD8+ T cells are decreased in the IL-17RA−/− mice at 10 dpi. n = 4/group; *, p = 0.02; **, p = 0.005. D, MPO activity is decreased in the lung homogenates, and trends lower in the BALF, of the IL-17RA−/− mice compared with wild-type (WT) controls (n = 5/group/time point). *, p = 0.03; **, p = 0.01. E, Levels of oxidized phospholipids, measured by ELISA, are lower in IL-17RA−/− mice than in BALB/c controls. n = 5/group/time point.***, p = 0.02.
Similar articles
- IL-17RA signaling reduces inflammation and mortality during Trypanosoma cruzi infection by recruiting suppressive IL-10-producing neutrophils.
Tosello Boari J, Amezcua Vesely MC, Bermejo DA, Ramello MC, Montes CL, Cejas H, Gruppi A, Acosta Rodríguez EV. Tosello Boari J, et al. PLoS Pathog. 2012;8(4):e1002658. doi: 10.1371/journal.ppat.1002658. Epub 2012 Apr 26. PLoS Pathog. 2012. PMID: 22577359 Free PMC article. - Regulation of gastric B cell recruitment is dependent on IL-17 receptor A signaling in a model of chronic bacterial infection.
Algood HM, Allen SS, Washington MK, Peek RM Jr, Miller GG, Cover TL. Algood HM, et al. J Immunol. 2009 Nov 1;183(9):5837-46. doi: 10.4049/jimmunol.0901206. Epub 2009 Oct 7. J Immunol. 2009. PMID: 19812196 Free PMC article. - Interleukin 17 (IL-17) manipulates mouse bone marrow- derived neutrophils in response to acute lung inflammation.
Chuammitri P, Wongsawan K, Pringproa K, Thanawongnuwech R. Chuammitri P, et al. Comp Immunol Microbiol Infect Dis. 2019 Dec;67:101356. doi: 10.1016/j.cimid.2019.101356. Epub 2019 Oct 3. Comp Immunol Microbiol Infect Dis. 2019. PMID: 31634721 - The IL-17A/IL-17RA axis in pulmonary defence and immunopathology.
Lorè NI, Bragonzi A, Cigana C. Lorè NI, et al. Cytokine Growth Factor Rev. 2016 Aug;30:19-27. doi: 10.1016/j.cytogfr.2016.03.009. Epub 2016 Mar 24. Cytokine Growth Factor Rev. 2016. PMID: 27033174 Review. - An Overview of Interleukin-17A and Interleukin-17 Receptor A Structure, Interaction and Signaling.
Krstic J, Obradovic H, Kukolj T, Mojsilovic S, Okic-Dordevic I, Bugarski D, Santibanez JF. Krstic J, et al. Protein Pept Lett. 2015;22(7):570-8. doi: 10.2174/0929866522666150520145554. Protein Pept Lett. 2015. PMID: 25990083 Review.
Cited by
- Cell-intrinsic regulation of phagocyte function by interferon lambda during pulmonary viral, bacterial super-infection.
Antos D, Parks OB, Duray AM, Abraham N, Michel JJ, Kupul S, Westcott R, Alcorn JF. Antos D, et al. PLoS Pathog. 2024 Aug 23;20(8):e1012498. doi: 10.1371/journal.ppat.1012498. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39178311 Free PMC article. - RSV enhances Staphylococcus aureus bacterial growth in the lung.
Rich HE, Bhutia S, Gonzales de Los Santos F, Entrup GP, Warheit-Niemi HI, Gurczynski SJ, Bame M, Douglas MT, Morris SB, Zemans RL, Lukacs NW, Moore BB. Rich HE, et al. Infect Immun. 2024 Oct 15;92(10):e0030424. doi: 10.1128/iai.00304-24. Epub 2024 Aug 16. Infect Immun. 2024. PMID: 39150268 - Intranasal Vaccination with Recombinant TLR2-Active Outer Membrane Vesicles Containing Sequential M2e Epitopes Protects against Lethal Influenza a Challenge.
Kannan N, Choi A, Rivera De Jesus MA, Wei PM, Sahler JM, Curley SM, August A, DeLisa MP, Whittaker GR, Putnam D. Kannan N, et al. Vaccines (Basel). 2024 Jun 29;12(7):724. doi: 10.3390/vaccines12070724. Vaccines (Basel). 2024. PMID: 39066362 Free PMC article. - Gut-derived immune cells and the gut-lung axis in ARDS.
Ziaka M, Exadaktylos A. Ziaka M, et al. Crit Care. 2024 Jul 4;28(1):220. doi: 10.1186/s13054-024-05006-x. Crit Care. 2024. PMID: 38965622 Free PMC article. Review. - Influenza A virus during pregnancy disrupts maternal intestinal immunity and fetal cortical development in a dose- and time-dependent manner.
Otero AM, Connolly MG, Gonzalez-Ricon RJ, Wang SS, Allen JM, Antonson AM. Otero AM, et al. Mol Psychiatry. 2024 Jul 3. doi: 10.1038/s41380-024-02648-9. Online ahead of print. Mol Psychiatry. 2024. PMID: 38961232
References
- Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31:S195–S199. - PubMed
- Lew TW, Kwek TK, Tai D, Earnest A, Loo S, Singh K, Kwan KM, Chan Y, Yim CF, Bek SL, et al. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290:374–380. - PubMed
- Beigel JH, Farrar J, Han AM, Hayden FG, Hyer R, de Jong MD, Lochindarat S, Nguyen TK, Nguyen TH, Tran TH, et al. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;353:1374–1385. - PubMed
- Tumpey TM, Basler CF, Aguilar PV, Zeng H, Solorzano A, Swayne DE, Cox NJ, Katz JM, Taubenberger JK, Palese P, Garcia-Sastre A. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science. 2005;310:77– 80. - PubMed
- Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, Ebihara H, Hatta Y, Kim JH, Halfmann P, Hatta M, et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–323. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous