Co-regulation of NF-kappaB and inflammasome-mediated inflammatory responses by myxoma virus pyrin domain-containing protein M013 - PubMed (original) (raw)
Co-regulation of NF-kappaB and inflammasome-mediated inflammatory responses by myxoma virus pyrin domain-containing protein M013
Masmudur M Rahman et al. PLoS Pathog. 2009 Oct.
Abstract
NF-kappaB and inflammasomes both play central roles in orchestrating anti-pathogen responses by rapidly inducing a variety of early-response cytokines and chemokines following infection. Myxoma virus (MYXV), a pathogenic poxvirus of rabbits, encodes a member of the cellular pyrin domain (PYD) superfamily, called M013. The viral M013 protein was previously shown to bind host ASC-1 protein and inhibit the cellular inflammasome complex that regulates the activation and secretion of caspase 1-regulated cytokines such as IL-1beta and IL-18. Here, we report that human THP-1 monocytic cells infected with a MYXV construct deleted for the M013L gene (vMyxM013-KO), in stark contrast to the parental MYXV, rapidly induce high levels of secreted pro-inflammatory cytokines like TNF, IL-6, and MCP-1, all of which are regulated by NF-kappaB. The induction of these NF-kappaB regulated cytokines following infection with vMyxM013-KO was also confirmed in vivo using THP-1 derived xenografts in NOD-SCID mice. vMyxM013-KO virus infection specifically induced the rapid phosphorylation of IKK and degradation of IkappaBalpha, which was followed by nuclear translocation of NF-kappaB/p65. Even in the absence of virus infection, transiently expressed M013 protein alone inhibited cellular NF-kappaB-mediated reporter gene expression and nuclear translocation of NF-kappaB/p65. Using protein/protein interaction analysis, we show that M013 protein also binds directly with cellular NF-kappaB1, suggesting a direct physical and functional linkage between NF-kappaB1 and ASC-1. We further demonstrate that inhibition of the inflammasome with a caspase-1 inhibitor did not prevent the induction of NF-kappaB regulated cytokines following infection with vMyxM013-KO virus, but did block the activation of IL-1beta. Thus, the poxviral M013 inhibitor exerts a dual immuno-subversive role in the simultaneous co-regulation of both the cellular inflammasome complex and NF-kappaB-mediated pro-inflammatory responses.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
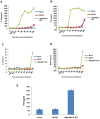
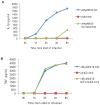
Figure 1. Kinetics of cytokine secretion in virus-infected THP-1 cells in vitro and in vivo.
THP-1 cells were differentiated for 12–18 h with PMA and then infected with MYXV or vMyxM013-KO viruses, at MOI of 3. Cell supernatants were collected at indicated time points to measure secretion of A) IL-1β, B) TNF, C) IL-6 and D) MCP-1 by ELISA. E) Level of human TNF secretion from virus infected THP-1 tumor xenografts implanted in NOD/SCID mice by s.c injection. Virus was injected intratumorally after 7 days of implantation and tissues were collected after 48 h of virus injection. TNF was detected by ELISA.
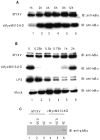
Figure 2. Activation of early inflammatory responses by vMyxM013-KO virus.
THP-1 cells were differentiated with PMA and infected with MYXV or vMyxM013-KO viruses, at MOI of 3 or treated with LPS (1 µg/ml) for indicated time points and harvested to prepare total cell extracts. Equal amounts of protein from uninfected, infected or LPS treated cells were analyzed by Western blot using IκBα (A and B) and phospho IκBα (pIκBα) (C) antibodies.
Figure 3. Phosphorylation of IKKα/β following virus infection.
THP-1 cells were differentiated with PMA and infected with MYXV or vMyxM013-KO viruses, at MOI of 3 for indicated time points and harvested to prepare total cell extracts. Equal amounts of protein loaded in SDS-PAGE to detect proteins by Western blot using A) phospho IKKα/β and B) IKKα/β specific antibodies. The stars indicate the phospho IKK protein bands.
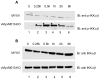
Figure 4. Activation of NF-κB1 and nuclear translocation of NF-κB p65 by vMyxM013-KO virus.
THP-1 cells were differentiated with PMA and infected with MYXV or vMyxM013-KO viruses at MOI of 3 for indicated time points and harvested to prepare total cell extracts and nuclear and cytoplasmic extracts. Equal amount of proteins were loaded in SDS-PAGE to detect the presence of A) NF-κB1; B) NF-κB p65 in the total cell extracts and C) NF-κB1 and p65 in the nuclear (top panels) and cytoplasmic (bottom panels) extracts. The proteins were detected using specific antibodies.
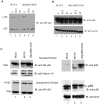
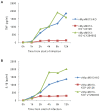
Figure 5. The effect of a specific caspase-1 inhibitor on cytokine secretion from virus-infected THP-1 cells.
THP-1 cells were differentiated with PMA and treated with caspase-1 inhibitor zVED-fmk (50 µM) for one hour and infected with MYXV or vMyxM013-KO viruses at MOI of 3. Cell supernatants were collected at indicated time points and tested for A) IL-1β and B) TNF by ELISA kit.
Figure 6. The effect of NF-κB signaling inhibitors on cytokine secretion from virus-infected THP-1 cells.
THP-1 cells were differentiated with PMA and treated with ERK1/2 inhibitor U0126 (20 µM) and PI3kinase inhibitor LY294002 (20 µM) for one hour and infected with MYXV or vMyxM013-KO viruses at MOI of 3. Cell supernatants were collected at indicated time points and tested for A) TNF and B) IL-1β by ELISA kit.
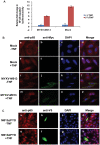
Figure 7. M013 protein alone suppresses NF-κB activity and nuclear translocation of p65.
(A) HeLa cells were co-transfected with equal amounts of pNF-κB-Luc and tk-Renilla-Luc along with pcDNA3.1M013L Myc/His or empty vector. At 48 h post-transfection, cells were either left untreated or treated with TNF (20 ng/ml) for 6 h and then assayed for both sea pansy and firefly luciferase activity. The presented values represent the average of three independent transfections. (B) Immunofluorescence and confocal microscopy analysis demonstrating the effect of M013 on TNF-induced nuclear translocation of NF-κB/p65 in HeLa cells. Mock (a–h) or M013L (i–p) transfected HeLa cells were labeled in the absence (a–d and i–l) or presence (e–h and m–p) of TNF treatment. (C) Immunofluorescence and confocal microscopy analysis demonstrating the effect of M013ΔPYD on TNF-induced translocation of NF-κB/p65 in HeLa cells. M013ΔPYD transfected HeLa cells were labeled in the absence (a–d) or presence (e–h) of TNF treatment.
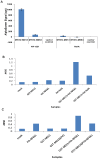
Figure 8. M013 protein interacts with NF-κB1 protein.
A) BSR-T7/5 cells were co-transfected with the pcDNA3.1Myc/His plasmid expressing Myc/His-tagged-NF-κB1 or RelA along with the pANT7_cGST plasmid expressing GST-tagged-M013, M063 or an empty pANT7_cGST plasmid. Following transfection, cell lysates were subjected to the Alphascreen protein-protein interaction assay using Nickel-coated donor beads and anti-GST-coated acceptor beads. The presented results represent the average of three independent transfections. Using in vitro TNT reactions GST-tagged M013 B) or M013ΔPYD C) and HA-tagged NF-κB1 or RelA were expressed alone or co-expressed from appropriate plasmids. The expressed proteins were bound to the anti-GST antibody coated 384 well plates. After washing the bound protein complexes were detected using anti-HA antibody and a HRP-coated secondary antibody. The binding of secondary antibody was detected by applying TMB substrate. The plate was then read at 450 nm using a multiwell plate reader.
Similar articles
- Myxoma virus M013 protein antagonizes NF-κB and inflammasome pathways via distinct structural motifs.
Garg RR, Jackson CB, Rahman MM, Khan AR, Lewin AS, McFadden G. Garg RR, et al. J Biol Chem. 2019 May 24;294(21):8480-8489. doi: 10.1074/jbc.RA118.006040. Epub 2019 Apr 2. J Biol Chem. 2019. PMID: 30940649 Free PMC article. - Hydrogen-Rich Saline Attenuated Subarachnoid Hemorrhage-Induced Early Brain Injury in Rats by Suppressing Inflammatory Response: Possible Involvement of NF-κB Pathway and NLRP3 Inflammasome.
Shao A, Wu H, Hong Y, Tu S, Sun X, Wu Q, Zhao Q, Zhang J, Sheng J. Shao A, et al. Mol Neurobiol. 2016 Jul;53(5):3462-3476. doi: 10.1007/s12035-015-9242-y. Epub 2015 Jun 20. Mol Neurobiol. 2016. PMID: 26091790 - Response of host inflammasomes to viral infection.
Chen IY, Ichinohe T. Chen IY, et al. Trends Microbiol. 2015 Jan;23(1):55-63. doi: 10.1016/j.tim.2014.09.007. Epub 2014 Oct 22. Trends Microbiol. 2015. PMID: 25456015 Review. - The role of the NLRP3 inflammasome in regulation of antiviral responses to influenza A virus infection.
Sarvestani ST, McAuley JL. Sarvestani ST, et al. Antiviral Res. 2017 Dec;148:32-42. doi: 10.1016/j.antiviral.2017.10.020. Epub 2017 Oct 31. Antiviral Res. 2017. PMID: 29097227 Review.
Cited by
- Unveiling the Mechanism of Protective Effects of Tanshinone as a New Fighter Against Cardiovascular Diseases: A Systematic Review.
Dabbaghi MM, Soleimani Roudi H, Safaei R, Baradaran Rahimi V, Fadaei MR, Askari VR. Dabbaghi MM, et al. Cardiovasc Toxicol. 2024 Sep 22. doi: 10.1007/s12012-024-09921-x. Online ahead of print. Cardiovasc Toxicol. 2024. PMID: 39306819 Review. - Biomaterials Functionalized with Inflammasome Inhibitors-Premises and Perspectives.
Vinţeler N, Feurdean CN, Petkes R, Barabas R, Boşca BA, Muntean A, Feștilă D, Ilea A. Vinţeler N, et al. J Funct Biomater. 2024 Jan 28;15(2):32. doi: 10.3390/jfb15020032. J Funct Biomater. 2024. PMID: 38391885 Free PMC article. Review. - Cross-species transmission and host range genes in poxviruses.
Yang CH, Song AL, Qiu Y, Ge XY. Yang CH, et al. Virol Sin. 2024 Apr;39(2):177-193. doi: 10.1016/j.virs.2024.01.007. Epub 2024 Jan 23. Virol Sin. 2024. PMID: 38272237 Free PMC article. Review. - Structural homology screens reveal host-derived poxvirus protein families impacting inflammasome activity.
Boys IN, Johnson AG, Quinlan MR, Kranzusch PJ, Elde NC. Boys IN, et al. Cell Rep. 2023 Aug 29;42(8):112878. doi: 10.1016/j.celrep.2023.112878. Epub 2023 Jul 25. Cell Rep. 2023. PMID: 37494187 Free PMC article. - Molluscum Contagiosum Virus Protein MC008 Targets NF-κB Activation by Inhibiting Ubiquitination of NEMO.
Phelan T, Lawler C, Pichlmair A, Little MA, Bowie AG, Brady G. Phelan T, et al. J Virol. 2023 Mar 30;97(3):e0010823. doi: 10.1128/jvi.00108-23. Epub 2023 Mar 14. J Virol. 2023. PMID: 36916940 Free PMC article.
References
- Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–208. - PubMed
- Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–96. - PubMed
- Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. - PubMed
- Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. - PubMed
- Baeuerle PA, Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988;242:540–546. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous