Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium - PubMed (original) (raw)
Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium
Ljiljana Milenkovic et al. J Cell Biol. 2009.
Abstract
The function of primary cilia depends critically on the localization of specific proteins in the ciliary membrane. A major challenge in the field is to understand protein trafficking to cilia. The Hedgehog (Hh) pathway protein Smoothened (Smo), a 7-pass transmembrane protein, moves to cilia when a ligand is received. Using microscopy-based pulse-chase analysis, we find that Smo moves through a lateral transport pathway from the plasma membrane to the ciliary membrane. Lateral movement, either via diffusion or active transport, is quite distinct from currently studied pathways of ciliary protein transport in mammals, which emphasize directed trafficking of Golgi-derived vesicles to the base of the cilium. We anticipate that this alternative route will be used by other signaling proteins that function at cilia. The path taken by Smo may allow novel strategies for modulation of Hh signaling in cancer and regeneration.
Figures
Figure 1.
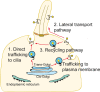
Three models for Hh-induced Smo transport to the primary cilium. (1) Direct trafficking from the Golgi to the base of the cilium. (2) Transport to the cell surface followed by lateral transport into the cilium. (3) Surface localization followed by internalization into a recycling pathway.
Figure 2.
Smo present on the cell surface translocates to the primary cilium after Shh stimulation. (A) Extracellular domains of Smo are recognized by anti-YFP (YFP tag), anti-SmoN (cysteine-rich domain), or the SNAP substrate (SNAP tag). An intracellular region of Smo is recognized by anti-SmoC. (B) Cell surface proteins were biotinylated, isolated on streptavidin beads, and examined for the presence of Smo or a control intracellular protein (p38) by immunoblotting. (C–E) Live YFP-Smo cells (Rohatgi et al., 2009) were exposed to anti-YFP (C and D) or anti-SmoN (E) according to the timeline shown to the left of each panel. (C) Insets (enlarged views of the boxed regions) show cilia visualized as shifted overlays of the color channels. (D) Intensity of Smo fluorescence at cilia, shown as fold increase, after treatment with Shh in cells pretreated with anti-YFP or anti-SmoC (control). Data indicate mean ± SEM. (E) Shh was added after cells were treated with anti-SmoN. Both the main panels and insets (enlarged views of the boxed regions) showing cilia are shifted overlays. Bars, 5 µm.
Figure 3.
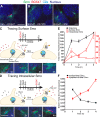
Pulse-chase labeling: surface-derived Smo enters cilia before intracellular Smo in response to Shh. All parts of the figure use cells expressing SNAP-tagged Smo. (A) Cells pretreated with Shh were labeled with the non–cell-permeable SNAP substrate BG-547. Shifted overlay shows that the BG-547 signal is coincident with cilia and the total SNAP-Smo protein detected with anti-SmoC. (B) Cells prelabeled with BG-547, washed, then treated with Shh show BG-547-labeled Smo localized at cilia. (C) To track Smo on cell surfaces, cells were treated as in B for the indicated periods of time. BG-547–labeled SNAP-Smo entered and then dissipated from the cilium. (D) To track intracellular Smo, SNAP-Smo on the cell surface was rendered invisible by treating cells with a non–cell-permeable block substrate (CBG block). After Shh treatment (times indicated), intracellular Smo that had translocated to the cilium was detected with BG-547 before fixation. Data from C and D were analyzed by plotting either the mean (±SEM) BG-547 and anti-SmoC fluorescence (E) or the mean BG-547/anti-SmoC fluorescence ratio (F) at various times after Shh treatment. Bars, 5 µm.
Figure 4.
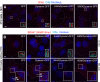
Dynamin-dependent endocytosis is not required for Smo localization in the cilia. (A) Dominant-negative dynamin mutants (K44A and I690K) did not block the translocation of endogenous Smo to cilia in Shh-treated (4 h) NIH3T3 cells. Transfected cells are outlined and were identified by GFP fluorescence (not shown for clarity), and insets show magnified views of cilia in the boxed regions (the ciliary marker acetylated tubulin is shown only in the insets as shifted overlays of two color channels). No Smo was detected at cilia in the absence of Shh in cells expressing any of the proteins (not depicted). (B) To establish that dominant-negative dynamin mutants can block the endocytosis of Smo, NIH3T3 cells were cotransfected with a SNAP-Smo gene and a gene encoding either wild-type dynamin or a dominant-negative dynamin. To follow the endocytosis of SNAP-Smo, cells were surface-labeled with nonpermeable BG-547, washed to remove unreacted BG-547, and fixed immediately (top) or after incubation at 37° for 60 min to allow internalization (bottom). Cells transfected with wild-type dynamin but not dominant-negative dynamin show clear evidence for the internalization of BG-547-labeled Smo into a perinuclear compartment (indicated by arrows). The lack of perinuclear accumulation indicates that dominant-negative dynamin mutants (K44A and I690K) blocked the endocytosis of overexpressed Smo. Despite these differences in internalization, Smo is present in cilia under all conditions. Bars, 5 µm.
Figure 5.
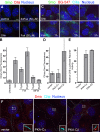
The cAMP–PKA pathway promotes Smo entry into primary cilia. (A and B) Smo is localized to primary cilia of NIH3T3 cells after treatment (4 h) with Shh or Fsk but not the inactive analogue dideoxy-Fsk (ddFsk). Representative images are shown in A, and mean (±SEM) ciliary Smo fluorescence is shown in B. (C) The labeling of surface SNAP-Smo (in SNAP-Smo cells) with non–cell-permeable BG-547 before treatment with Shh or Fsk shows that Fsk, like Shh, induces accumulation of BG-547-labeled Smo (surface Smo) in cilia by 1 h. The experiment timeline is as in Fig. 3 C. Images are shifted overlays of the color channels. (D) The mean (±SEM) Smo fluorescence at cilia of transfected cells from the experiment shown in F. (E) The same PKA-Cα and PKA-Cβ constructs used in C were tested for their abilities to induce a cAMP response element–linked luciferase reporter. (F) Cells transfected with genes encoding either the α or β catalytic subunit of PKA were stained to show cilia and Smo. Broken lines demarcate transfected cells. Insets are magnified shifted overlays (indicated by the boxed regions) of two color channels. Bars, 5 µm.
Similar articles
- Smoothened adopts multiple active and inactive conformations capable of trafficking to the primary cilium.
Wilson CW, Chen MH, Chuang PT. Wilson CW, et al. PLoS One. 2009;4(4):e5182. doi: 10.1371/journal.pone.0005182. Epub 2009 Apr 13. PLoS One. 2009. PMID: 19365551 Free PMC article. - Gli2 trafficking links Hedgehog-dependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus.
Kim J, Kato M, Beachy PA. Kim J, et al. Proc Natl Acad Sci U S A. 2009 Dec 22;106(51):21666-71. doi: 10.1073/pnas.0912180106. Epub 2009 Dec 8. Proc Natl Acad Sci U S A. 2009. PMID: 19996169 Free PMC article. - Small molecule inhibitors of Smoothened ciliary localization and ciliogenesis.
Wu VM, Chen SC, Arkin MR, Reiter JF. Wu VM, et al. Proc Natl Acad Sci U S A. 2012 Aug 21;109(34):13644-9. doi: 10.1073/pnas.1207170109. Epub 2012 Aug 3. Proc Natl Acad Sci U S A. 2012. PMID: 22864913 Free PMC article. - Mechanisms of Smoothened Regulation in Hedgehog Signaling.
Zhang J, Liu Z, Jia J. Zhang J, et al. Cells. 2021 Aug 20;10(8):2138. doi: 10.3390/cells10082138. Cells. 2021. PMID: 34440907 Free PMC article. Review. - Hedgehog signaling pathway: a novel model and molecular mechanisms of signal transduction.
Gorojankina T. Gorojankina T. Cell Mol Life Sci. 2016 Apr;73(7):1317-32. doi: 10.1007/s00018-015-2127-4. Epub 2016 Jan 13. Cell Mol Life Sci. 2016. PMID: 26762301 Free PMC article. Review.
Cited by
- Arih2 regulates Hedgehog signaling through smoothened ubiquitylation and ER-associated degradation.
Lv B, Zhang XO, Pazour GJ. Lv B, et al. J Cell Sci. 2022 Aug 15;135(16):jcs260299. doi: 10.1242/jcs.260299. Epub 2022 Aug 22. J Cell Sci. 2022. PMID: 35899529 Free PMC article. - From inflammation to gastric cancer - the importance of Hedgehog/GLI signaling in Helicobacter pylori-induced chronic inflammatory and neoplastic diseases.
Wessler S, Krisch LM, Elmer DP, Aberger F. Wessler S, et al. Cell Commun Signal. 2017 Apr 20;15(1):15. doi: 10.1186/s12964-017-0171-4. Cell Commun Signal. 2017. PMID: 28427431 Free PMC article. Review. - Single-molecule imaging in the primary cilium.
Weiss LE, Love JF, Yoon J, Comerci CJ, Milenkovic L, Kanie T, Jackson PK, Stearns T, Gustavsson AK. Weiss LE, et al. Methods Cell Biol. 2023;176:59-83. doi: 10.1016/bs.mcb.2023.01.003. Epub 2023 Feb 24. Methods Cell Biol. 2023. PMID: 37164543 Free PMC article. - Progress in ciliary ion channel physiology.
Pablo JL, DeCaen PG, Clapham DE. Pablo JL, et al. J Gen Physiol. 2017 Jan;149(1):37-47. doi: 10.1085/jgp.201611696. Epub 2016 Dec 20. J Gen Physiol. 2017. PMID: 27999145 Free PMC article. Review. - In Vitro Modeling Using Ciliopathy-Patient-Derived Cells Reveals Distinct Cilia Dysfunctions Caused by CEP290 Mutations.
Shimada H, Lu Q, Insinna-Kettenhofen C, Nagashima K, English MA, Semler EM, Mahgerefteh J, Cideciyan AV, Li T, Brooks BP, Gunay-Aygun M, Jacobson SG, Cogliati T, Westlake CJ, Swaroop A. Shimada H, et al. Cell Rep. 2017 Jul 11;20(2):384-396. doi: 10.1016/j.celrep.2017.06.045. Cell Rep. 2017. PMID: 28700940 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- HHMI/Howard Hughes Medical Institute/United States
- K99 CA129174/CA/NCI NIH HHS/United States
- R00 CA129174/CA/NCI NIH HHS/United States
- 5K99CA129174/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous