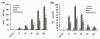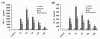Histone deacetylase inhibitor, butyrate, attenuates lipopolysaccharide-induced acute lung injury in mice - PubMed (original) (raw)
Histone deacetylase inhibitor, butyrate, attenuates lipopolysaccharide-induced acute lung injury in mice
Yun-Feng Ni et al. Respir Res. 2010.
Abstract
Background: Histone deacetylase (HDAC) inhibitors, developed as promising anti-tumor drugs, exhibit their anti-inflammatory properties due to their effects on reduction of inflammatory cytokines.
Objective: To investigate the protective effect of butyrate, a HDAC inhibitor, on lipopolysaccharide (LPS)-induced acute lung injury (ALI) in mice.
Methods: ALI was induced in Balb/c mice by intratracheally instillation of LPS (1 mg/kg). Before 1 hour of LPS administration, the mice received butyrate (10 mg/kg) orally. The animals in each group were sacrificed at different time point after LPS administration. Pulmonary histological changes were evaluated by hematoxylin-eosin stain and lung wet/dry weight ratios were observed. Concentrations of interleukin (IL)-1beta and tumor necrosis factor (TNF)-alpha in bronchoalveolar lavage fluid (BALF) and concentrations of nitric oxide (NO) and myeloperoxidase (MPO) activity in lung tissue homogenates were measured by enzyme-linked immunosorbent assay (ELISA). Expression of nuclear factor (NF)-kappaB p65 in cytoplasm and nucleus was determined by Western blot analysis respectively.
Results: Pretreatment with butyrate led to significant attenuation of LPS induced evident lung histopathological changes, alveolar hemorrhage, and neutrophils infiltration with evidence of reduced MPO activity. The lung wet/dry weight ratios, as an index of lung edema, were reduced by butyrate administration. Butyrate also repressed the production of TNF-alpha, IL-1beta and NO. Furthermore, the expression of NF-kappaB p65 in nucleus was markedly suppressed by butyrate pretreatment.
Conclusions: Butyrate had a protective effect on LPS-induced ALI, which may be related to its effect on suppression of inflammatory cytokines production and NF-kappaB activation.
Figures
Figure 1
Effect of butyrate on MPO activity and NO concentrations in lung tissues of mice with ALI. A. The MPO activity in lung tissues at each time point after LPS administration and the effect of butyrate pretreatment. B. The concentrations of NO in lung tissues at each time point after LPS administration and the effect of butyrate pretreatment. Data are expressed as mean ± SEM, *P < 0.05 vs. control and butyrate group; #P < 0.05 vs. LPS group.
Figure 2
Effect of butyrate on the concentrations of TNF-α and IL-1β in BALF of mice with ALI. A. The concentrations of TNF-α in BALF at each time point after LPS administration and the effect of butyrate pretreatment. B. The concentrations of IL-1β in BALF at each time point after LPS administration and the effect of butyrate pretreatment. Data are expressed as mean ± SEM, *P < 0.05 vs. control and butyrate group; #P < 0.05 vs. LPS group.
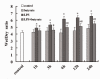
Figure 3
Effect of butyrate on the lung edema of mice with ALI. At each time point after LPS administration, pretreatment of butyrate decreased lung wet/dry ratios markedly. Data are expressed as mean ± SEM, *P < 0.05 vs. control and butyrate group; #P < 0.05 vs. LPS group.
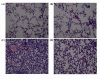
Figure 4
Effect of butyrate on the pulmonary histopathological changes of mice with ALI. Lung sections stained with hematoxylin-eosin from 24 hours after LPS administration revealed pulmonary histopathological changes (original magnification ×200). A. control group: normal structure. B. butyrate group: same as control group. C. LPS group: alveolar wall thickness, hemorrhage, alveolus collapse and obvious inflammatory cells infiltration. D. LPS + butyrate group: minor histopathological changes compared with LPS group.
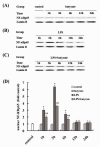
Figure 5
Effect of butyrate on the nuclear expression of NF-κB in lung tissues of mice with ALI. A., B. and C. Representative Western blots showed the nuclear expression of NF-κB p65 in lung tissues in different groups. D. Mean ± SEM NF-κB p65 optical densitometry from different groups. Pretreatment of butyrate significantly repressed the expression of NF-κB p65 in nucleus at 1, 3 and 6 hours after LPS administration. Data are expressed as mean ± SEM, *P < 0.05 vs. control and butyrate group; #P < 0.05 vs. LPS group.
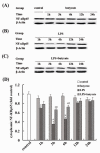
Figure 6
Effect of butyrate on the cytoplasmic expression of NF-κB in lung tissues of mice with ALI. A., B. and C. Representative Western blots showed the cytoplasmic expression of NF-κB p65 in lung tissues in different groups. D. Mean ± SEM NF-κB p65 optical densitometry from different groups. The expression of NF-κB p65 in cytoplasm was significantly reduced by LPS administration and these changes were inhibited by butyrate pretreatment. Data are expressed as mean ± SEM, *P < 0.05 vs. control and butyrate group; #P < 0.05 vs. LPS group.
Similar articles
- Ulinastatin inhibits the inflammation of LPS-induced acute lung injury in mice via regulation of AMPK/NF-κB pathway.
Li W, Qiu X, Jiang H, Zhi Y, Fu J, Liu J. Li W, et al. Int Immunopharmacol. 2015 Dec;29(2):560-567. doi: 10.1016/j.intimp.2015.09.028. Epub 2015 Oct 21. Int Immunopharmacol. 2015. PMID: 26481965 - Glycyrrhizin treatment is associated with attenuation of lipopolysaccharide-induced acute lung injury by inhibiting cyclooxygenase-2 and inducible nitric oxide synthase expression.
Ni YF, Kuai JK, Lu ZF, Yang GD, Fu HY, Wang J, Tian F, Yan XL, Zhao YC, Wang YJ, Jiang T. Ni YF, et al. J Surg Res. 2011 Jan;165(1):e29-35. doi: 10.1016/j.jss.2010.10.004. Epub 2010 Nov 10. J Surg Res. 2011. PMID: 21074783 - Crocin attenuates lipopolysacchride-induced acute lung injury in mice.
Wang J, Kuai J, Luo Z, Wang W, Wang L, Ke C, Li X, Ni Y. Wang J, et al. Int J Clin Exp Pathol. 2015 May 1;8(5):4844-50. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26191176 Free PMC article. - Kegan Liyan oral liquid ameliorates lipopolysaccharide-induced acute lung injury through inhibition of TLR4-mediated NF-κB signaling pathway and MMP-9 expression.
Zhang X, Sun CY, Zhang YB, Guo HZ, Feng XX, Peng SZ, Yuan J, Zheng RB, Chen WP, Su ZR, Huang XD. Zhang X, et al. J Ethnopharmacol. 2016 Jun 20;186:91-102. doi: 10.1016/j.jep.2016.03.057. Epub 2016 Mar 30. J Ethnopharmacol. 2016. PMID: 27036629 - Protective effect of Cordyceps sinensis extract on lipopolysaccharide-induced acute lung injury in mice.
Fu S, Lu W, Yu W, Hu J. Fu S, et al. Biosci Rep. 2019 Jun 25;39(6):BSR20190789. doi: 10.1042/BSR20190789. Print 2019 Jun 28. Biosci Rep. 2019. PMID: 31186277 Free PMC article.
Cited by
- Fructose-1,6-bisphosphate protects against Zymosan-induced acute lung injury in mice.
Santos RC, Moresco RN, Peña Rico MA, Susperregui AR, Rosa JL, Bartrons R, Ventura F, Mário DN, Alves SH, Tatsch E, Kober H, de Mello RO, Scherer P, de Oliveira JR. Santos RC, et al. Inflammation. 2012 Jun;35(3):1198-203. doi: 10.1007/s10753-012-9429-6. Inflammation. 2012. PMID: 22327861 - Prospects for clinical applications of butyrate-producing bacteria.
Zhu LB, Zhang YC, Huang HH, Lin J. Zhu LB, et al. World J Clin Pediatr. 2021 Sep 9;10(5):84-92. doi: 10.5409/wjcp.v10.i5.84. eCollection 2021 Sep 9. World J Clin Pediatr. 2021. PMID: 34616650 Free PMC article. Review. - Valproic acid: an anticonvulsant drug with potent antinociceptive and anti-inflammatory properties.
Ximenes JC, de Oliveira Gonçalves D, Siqueira RM, Neves KR, Santos Cerqueira G, Correia AO, Félix FH, Leal LK, de Castro Brito GA, da Graça Naffah-Mazzacorati M, Viana GS. Ximenes JC, et al. Naunyn Schmiedebergs Arch Pharmacol. 2013 Jul;386(7):575-87. doi: 10.1007/s00210-013-0853-4. Epub 2013 Apr 14. Naunyn Schmiedebergs Arch Pharmacol. 2013. PMID: 23584602 - Short-chain fatty acids suppress lipopolysaccharide-induced production of nitric oxide and proinflammatory cytokines through inhibition of NF-κB pathway in RAW264.7 cells.
Liu T, Li J, Liu Y, Xiao N, Suo H, Xie K, Yang C, Wu C. Liu T, et al. Inflammation. 2012 Oct;35(5):1676-84. doi: 10.1007/s10753-012-9484-z. Inflammation. 2012. PMID: 22669487 - Anti-inflammatory effects of novel curcumin analogs in experimental acute lung injury.
Zhang Y, Liang D, Dong L, Ge X, Xu F, Chen W, Dai Y, Li H, Zou P, Yang S, Liang G. Zhang Y, et al. Respir Res. 2015 Mar 24;16(1):43. doi: 10.1186/s12931-015-0199-1. Respir Res. 2015. PMID: 25889862 Free PMC article.
References
- Ulich TR, Fann MJ, Patterson PH, Williams JH, Samal B, Castillo JD, Yin S, Guo K, Remick DG. Intratracheal injection of LPS and cytokines. V. LPS induces expression of LIF and LIF inhibits acute inflammation. Am J Physiol Lung Cell Mol Physiol. 1994;267:442–446. - PubMed
- Blackwell TS, Christman JW. The role of nuclear factor-κB in cytokine gene regulation. Am J Respir Cell Mol Biol. 1997;17:3–9. - PubMed
- Kelly WK, Richon VM, O'Connor O, Curley T, MacGregor CB, Tong W, Klang M, Schwartz L, Richardson S, Rosa E, Drobnjak M, Cordon CC, Chiao JH, Rifkind R, Marks PA, Scher H. Phase I clinical trial of histone deacetylase inhibitor: suberoylanilide hydroxamic acid administered intravenously. Clin Cancer Res. 2003;9:3578–3588. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous