KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma - PubMed (original) (raw)
Review
. 2010 Oct;10(10):683-95.
doi: 10.1038/nrc2899. Epub 2010 Sep 3.
Affiliations
- PMID: 20814421
- PMCID: PMC4085546
- DOI: 10.1038/nrc2899
Review
KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma
John P Morris 4th et al. Nat Rev Cancer. 2010 Oct.
Abstract
Pancreatic ductal adenocarcinoma (PDAC) is characterized by near-universal mutations in KRAS and frequent deregulation of crucial embryonic signalling pathways, including the Hedgehog (Hh) and Wnt-β-catenin cascades. The creation of mouse models that closely resemble the human disease has provided a platform to better understand when and in which cell types these pathways are misregulated during PDAC development. Here we examine the central part that KRAS plays in the biology of PDAC, and how the timing and location of Hh and Wnt-β-catenin signalling dictate the specification and oncogenic properties of PDAC.
Figures
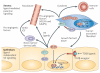
Figure 1. KRAS is a master regulator of pancreatic ductal adenocarcinoma initiation and progression
Constitutively active KRAS (caused by KrasG12D or KrasG12V mutations) is sufficient to initiate the development of pancreatic intraepithelial neoplasia (PanIN) and pancreatic ductal adenocarcinoma (PDAC). PanINs are classified into three stages of increasing cellular atypia and, in humans, have been found to possess increasing numbers of mutations (common mutations are indicated in boxes). Changes in the epithelium are matched by desmoplastic changes in the stroma. In mouse models, the human PanIN spectrum followed by progression to PDAC has been recapitulated by activating mutant KRAS in embryonic pancreatic progenitors. Eliminating tumour suppressors commonly inactivated in the human disease dramatically decreases PDAC latency (a limited set of examples is indicated). Mouse models in which KRAS is activated specifically in some adult cell types have shown that both acini and insulin-positive cells can give rise to PanINs and, in some cases, PDAC depending on tissue damage and tumour suppressor inactivation. For these cell types, reprogramming into a ‘ductal’ cell type is required to assume the PanIN–PDAC lineage. Question marks are shown for centroacinar and duct cells as they have not been specifically assessed for their ability to be reprogrammed into a lineage capable of becoming PDAC under the control of KRAS. However, until specific targeting has been achieved, they cannot be ruled out as sources of the precursor–PDAC lineage. Figure is modified, with permission, from REF. 128 © (2000) American Association of Cancer Research.
Figure 2. Hedgehog signalling in pancreatic ductal adenocarcinoma
Although the pancreatic ductal adenocarcinoma (PDAC) epithelium overexpresses Hedgehog (Hh), ligand-dependent canonical signalling is activated in stromal cells, including cancer-associated fibroblasts, infiltrating bone marrow-derived cells and subsets of endothelial cells, through the patched (PTC)–smoothened (SMO) axis. In turn, these cells directly proliferate or produce factors that might enhance tumour cell growth (potentially through secreted growth factors or by changing extracellular matrix (ECM) composition) and angiogenesis in a paracrine fashion (the factors that have been identified are indicated). Furthermore, cancer-associated fibroblasts and other Hh-responsive cells might produce cytokines and other molecules that communicate with infiltrating immune cells. Conversely, autocrine activation through this canonical pathway does not seem to occur in the tumour epithelium. Gli activity is maintained in part by activation of GLI1 through alternative signalling pathways, such as mutant KRAS expression and transforming growth factor-β (TGFβ) signalling. ANGPT1, angiopoietin 1; IGF, insulin-like growth factor; MMP9, matrix metalloproteinase 9; VEGFA, vascular endothelial growth factor A.
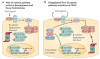
Figure 3. Canonical Wnt–β-catenin signalling in pancreatic ductal adencocarcinoma
a | In normal development, canonical Wnt–β-catenin signalling depends on secreted ligands (Wnt ligands) that activate receptors (Frizzled (Fzd)–Lrp complex) that block the proteosomal degradation of β-catenin promoted by the destruction complex (comprising adeomatous polyposis coli (APC), axin, glycogen synthase kinase 3β (GSK3β) and other proteins) through activation of Dishevelled (Dvl). b | β-catenin accumulation is frequently observed in pancreatic ductal adenocarcinoma (PDAC). Accumulated β-catenin can translocate into the nucleus and activate target genes in concert with TCF/LEF co-factors. Presently, the dominant mechanism of persistent β-catenin accumulation and activity in PDAC is unclear. There is evidence for both autocrine (owing to epithelial-derived Wnt ligands) and cell-autonomous activation (through Gli signalling and genes such as ataxia telangiectasia group D-associated (ATDC), which activates Dvl). There may also be contributions from the stromal cells and extracellular matrix that may promote β-catenin accumulation.
Figure 4. Crucial temporal thresholds of developmental signalling pathways and KRAS activity allow pancreatic epithelial neoplasia — pancreatic ductal adenocarcinoma initiation and progression
KRAS activity above a crucial threshold can drive differentiated pancreatic cells (acinar cells, for example) into a de-differentiated, ductal state that persists in pancreatic epithelial neoplasia (PanIN) and pancreatic ductal adenocarcinoma (PDAC). For these de-differentiated ductal cells to become PanINs, β-catenin signalling must be maintained below a crucial low level. However, once the PanIN state is established, β-catenin signalling is reactivated in parallel with increasing expression of Hedgehog (Hh) ligand that activates target genes in stromal cells of the developing desmoplastic response. Gli activity in the developing tumour epithelium emerges independently of autocrine stimulation. Although Gli activity is probably active in PanINs, its role in the progression from PanIN to PDAC is unknown. Finally, Gli activity is probably important for PDAC maintenance. Figure is modified, with permission, from REF. 128 © (2000) American Association of Cancer Research.
Similar articles
- Distinct functions of three Wnt proteins control mirror-symmetric organogenesis in the C. elegans gonad.
So S, Asakawa M, Sawa H. So S, et al. Elife. 2024 Nov 1;13:e103035. doi: 10.7554/eLife.103035. Elife. 2024. PMID: 39485276 Free PMC article. - KRAS-mediated upregulation of CIP2A promotes suppression of PP2A-B56α to initiate pancreatic cancer development.
Tinsley SL, Chianis ERD, Shelley RA, Mall GK, Dhiman A, Baral G, Kothandaraman H, Thoma MC, English IA, Daniel CJ, Acosta LCS, Solorio L, Atallah Lanman N, Pasca di Magliano M, Narla G, Dykhuizen EC, Sears RC, Allen-Petersen BL. Tinsley SL, et al. Oncogene. 2024 Dec;43(50):3673-3687. doi: 10.1038/s41388-024-03196-w. Epub 2024 Oct 23. Oncogene. 2024. PMID: 39443726 - Dosage-dependent hedgehog signals integrated with Wnt/beta-catenin signaling regulate external genitalia formation as an appendicular program.
Miyagawa S, Moon A, Haraguchi R, Inoue C, Harada M, Nakahara C, Suzuki K, Matsumaru D, Kaneko T, Matsuo I, Yang L, Taketo MM, Iguchi T, Evans SM, Yamada G. Miyagawa S, et al. Development. 2009 Dec;136(23):3969-78. doi: 10.1242/dev.039438. Development. 2009. PMID: 19906864 Free PMC article. - Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice.
Grigoryan T, Wend P, Klaus A, Birchmeier W. Grigoryan T, et al. Genes Dev. 2008 Sep 1;22(17):2308-41. doi: 10.1101/gad.1686208. Genes Dev. 2008. PMID: 18765787 Free PMC article. Review. - Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
Cited by
- Phase Ib Study of Wnt Inhibitor Ipafricept with Gemcitabine and nab-paclitaxel in Patients with Previously Untreated Stage IV Pancreatic Cancer.
Dotan E, Cardin DB, Lenz HJ, Messersmith W, O'Neil B, Cohen SJ, Denlinger CS, Shahda S, Astsaturov I, Kapoun AM, Brachmann RK, Uttamsingh S, Stagg RJ, Weekes C. Dotan E, et al. Clin Cancer Res. 2020 Oct 15;26(20):5348-5357. doi: 10.1158/1078-0432.CCR-20-0489. Epub 2020 Jul 21. Clin Cancer Res. 2020. PMID: 32694153 Free PMC article. Clinical Trial. - Metabolic determinants of tumour initiation.
Brunner JS, Finley LWS. Brunner JS, et al. Nat Rev Endocrinol. 2023 Mar;19(3):134-150. doi: 10.1038/s41574-022-00773-5. Epub 2022 Nov 29. Nat Rev Endocrinol. 2023. PMID: 36446897 Free PMC article. Review. - Genetic markers of malignant transformation in intraductal papillary mucinous neoplasm of the pancreas: a meta-analysis.
Nissim S, Idos GE, Wu B. Nissim S, et al. Pancreas. 2012 Nov;41(8):1195-205. doi: 10.1097/MPA.0b013e3182580fb4. Pancreas. 2012. PMID: 22750975 Free PMC article. - TGF-β1 promotes acinar to ductal metaplasia of human pancreatic acinar cells.
Liu J, Akanuma N, Liu C, Naji A, Halff GA, Washburn WK, Sun L, Wang P. Liu J, et al. Sci Rep. 2016 Aug 3;6:30904. doi: 10.1038/srep30904. Sci Rep. 2016. PMID: 27485764 Free PMC article. - Molecular alterations in pancreatic tumors.
Visani M, Acquaviva G, De Leo A, Sanza V, Merlo L, Maloberti T, Brandes AA, Franceschi E, Di Battista M, Masetti M, Jovine E, Fiorino S, Pession A, Tallini G, de Biase D. Visani M, et al. World J Gastroenterol. 2021 Jun 7;27(21):2710-2726. doi: 10.3748/wjg.v27.i21.2710. World J Gastroenterol. 2021. PMID: 34135550 Free PMC article. Review.
References
- Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–1249. - PubMed
- Corcoran RB, Scott MP. A mouse model for medulloblastoma and basal cell nevus syndrome. J. Neurooncol. 2001;53:307–318. - PubMed
- Romer JT, et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1+/−p53−/− mice. Cancer Cell. 2004;6:229–240. - PubMed
- Taketo MM, Edelmann W. Mouse models of colon cancer. Gastroenterology. 2009;136:780–798. - PubMed
- Habbe N, Langer P, Sina-Frey M, Bartsch DK. Familial pancreatic cancer syndromes. Endocrinol. Metab. Clin. North Am. 2006;35:417–430. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous