Countervailing modulation of Ih by neuropeptide Y and corticotrophin-releasing factor in basolateral amygdala as a possible mechanism for their effects on stress-related behaviors - PubMed (original) (raw)
Countervailing modulation of Ih by neuropeptide Y and corticotrophin-releasing factor in basolateral amygdala as a possible mechanism for their effects on stress-related behaviors
Chantelle J Giesbrecht et al. J Neurosci. 2010.
Abstract
Stress and anxiety-related behaviors controlled by the basolateral amygdala (BLA) are regulated in vivo by neuropeptide Y (NPY) and corticotrophin-releasing factor (CRF): NPY produces anxiolytic effects, whereas CRF produces anxiogenic effects. These opposing actions are likely mediated via regulation of excitatory output from the BLA to afferent targets. In these studies, we examined mechanisms underlying the effects of NPY and CRF in the BLA using whole-cell patch-clamp electrophysiology in rat brain slices. NPY, even with tetrodotoxin present, caused a dose-dependent membrane hyperpolarization in BLA pyramidal neurons. The hyperpolarization resulted in the inhibition of pyramidal cells, despite arising from a reduction in a voltage-dependent membrane conductance. The Y(1) receptor agonist, F(7)P(34) NPY, produced a similar membrane hyperpolarization, whereas the Y(1) antagonist, BIBO3304 [(R)-N-[[4-(aminocarbonylaminomethyl)-phenyl]methyl]-N(2)-(diphenylacetyl)-argininamide trifluoroacetate], blocked the effect of NPY. The NPY-inhibited current was identified as I(h), which is active at and hyperpolarized to rest. Responses to NPY were occluded by either Cs(+) or ZD7288 (4-ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride), but unaffected by the G(IRK)-preferring blockers Ba(2+) and SCH23390 [(R)-(+)-7-chloro-8-hydroxy-3-methyl-l-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride]. Application of CRF, with or without TTX present, depolarized NPY-sensitive BLA pyramidal neurons, resulting from an increase in I(h). Electrophysiological and immunocytochemical data were consistent with a major role for the HCN1 subunit. Our results indicate that NPY, via Y(1) receptors, directly inhibits BLA pyramidal neurons by suppressing a postsynaptic I(h), whereas CRF enhances resting I(h), causing an increased excitability of BLA pyramidal neurons. The opposing actions of these two peptides on the excitability of BLA output cells are consistent with the observed behavioral actions of NPY and CRF in the BLA.
Figures
Figure 1.
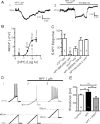
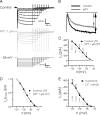
NPY inhibits BLA pyramidal neurons. A1, Current-clamp voltage recording (10 min continuous trace) of the hyperpolarization caused in a single BLA pyramidal neuron by application of NPY (1 μ
m
). A2, NPY has a similar effect when applied in the presence of 500 n
m
TTX in a different BLA neuron. B, Concentration–response curve for the hyperpolarizing effect of NPY on membrane potential (ΔRMP). Concentrations ranged from 30 n
m
to 3 μ
m
, with NPY (1 μ
m
) having a near-maximal effect. Values next to each point denote the number of cells observed under that condition; significance values represent differences from zero effect. Effect of the Y1 agonist F7P34 NPY (1 μ
m
) is shown as a gray square. C, Summary bar graph comparing the response to NPY receptor-selective agonists or the effect of NPY in the presence of receptor-selective antagonists against the response to NPY itself in BLA pyramidal cells. Values within each bar represent number of cells per condition. Error bars represent mean ± SEM. D, Effect of NPY (1 μ
m
) on BLA neuron excitability. Membrane potential responses to current ramps (bottom traces) in control (D1) and in NPY (D2). D3, Increased firing response in the presence of NPY to current ramp in D1 and D2 superimposed on steady depolarizing current (bottom trace) returning cell to control membrane potential. E, Rheobase determined for BLA pyramidal cells in control, in the presence of 1 μ
m
NPY, and depolarized to the control membrane potential with NPY present. Error bars in this and all subsequent figures represent mean ± SEM. Numbers of neurons tested under each condition for this and all other bar graphs are indicated in the bar. Significance levels for statistical tests in this and all other figures as described are denoted as follows: *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 2.
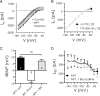
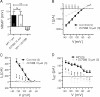
NPY reduces a steady-state current in BLA pyramidal cells by a postsynaptic action independent of GABAA receptors. A, Raw steady-state current responses to a slow voltage ramp compared in a single BLA neuron in control (black) with 1 μ
m
NPY present (dark gray) and after washout (light gray). B, Comparison of steady-state current–voltage relationships from BLA pyramidal cells at potentials indicated without and with TTX present (n = 12). The dotted line represents zero current. C, Comparison, in the same neurons, of membrane potential change with NPY (1 μ
m
) alone, bicuculline (10 μ
m
) alone, and NPY in the presence of bicuculline (n = 9). D, Net current change (_I_NET) caused by NPY in the absence or presence of bicuculline in the same neurons (n = 9). ns, No significant difference.
Figure 3.
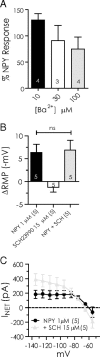
The inhibitory actions of NPY do not involve a GIRK. A, Response to NPY compared at −120 mV before and after treatment with different concentrations of Ba2+ (10, 30, and 100 μ
m
). B, Comparison of hyperpolarization by NPY (1 μ
m
) alone, SCH23390 (10 μ
m
) alone, and NPY after SCH23390 pretreatment. C, Comparison of net current blocked by NPY before and after SCH23390 pretreatment. Differences were not significant (n = 5).
Figure 4.
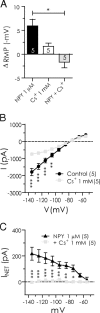
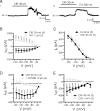
The response to NPY in BLA pyramidal cells is occluded by Cs+. A, Magnitude of resting membrane potential change with NPY (1 μ
m
) alone, Cs+ (1 m
m
) alone, and NPY after Cs+ pretreatment. B, Steady-state membrane current response to voltage ramp (n = 5) is significantly reduced in presence of Cs+ alone. C, Voltage ramp summary comparing net current blocked by NPY in the absence and presence of Cs+. Cs+ completely occluded the effect of NPY (n = 5).
Figure 5.
NPY reduces the amplitude of _I_h in BLA pyramidal cells. A, _I_h isolated (between −65 and −125 mV) with hyperpolarizing voltage steps in control (top) and with 1 μ
m
NPY (middle). Cells were held at −55 mV and hyperpolarizing steps applied (bottom traces). B, Current responses to voltage step from −55 to −125 mV illustrating reduction in _I_h amplitude with NPY (1 μ
m
). C, Comparison of mean _I_h amplitude at potentials between −125 and −75 mV in control and with NPY application (n = 77). _I_h amplitude for each cell and condition was determined as illustrated in B. D, NPY induced a small leftward shift in the activation curve for _I_h (n = 77). Data were normalized to the maximum _I_h available for each condition, transformed to absolute values, and fitted to a nonlinear regression. Voltage shift for _I_h was determined at 50% I/_I_max. E, Cs+ strongly suppresses _I_h in BLA pyramidal cells, determined using the same hyperpolarizing voltage step protocol as described in A (n = 5).
Figure 6.
The _I_h blocker ZD7288 hyperpolarizes BLA pyramidal neurons and occludes the actions of NPY. A, Net membrane potential hyperpolarization compared in the same neurons with NPY (1 μ
m
), ZD7288 (10 μ
m
), and NPY after ZD7288 pretreatment (n = 5). B, Total steady-state membrane current is reduced by ZD7288 in BLA pyramidal cells (n = 5). C, ZD7288 sharply reduces _I_h amplitude (n = 5). D, Net current caused by NPY in the absence and the presence of ZD7288 (n = 5).
Figure 7.
CRF depolarizes NPY-sensitive BLA neurons by enhancement of _I_h. A1, A2, Change in membrane potential caused by 30 n
m
CRF in control (A1) or in the presence of TTX (A2) (recordings are from different neurons). B, Changes in steady-state current caused by CRF and NPY application in the same neurons (n = 5). C, Increase in _I_h amplitude caused by CRF. D, CRF-sensitive current is blocked by ZD7288 (n = 4). E, NPY blocks CRF effects in CRF-sensitive neurons (n = 3).
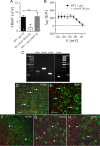
Figure 8.
NPY-sensitive H-current is likely mediated by HCN1 subunits. A, NPY-mediated change in resting membrane potential is unaltered by pretreatment with the membrane-permeant cAMP analog, db-cAMP (30 μ
m
). B, Net current caused by NPY is unaltered in the presence of db-cAMP (cAMP, 30 μ
m
; n = 5). C, Gel electrophoresis of RT-PCR products showing specific bands for HCN1 (288 bp), HCN2 (370 bp), HCN3 (233 bp), and HCN4 (172 bp) mRNA expression in BLA tissue. cDNA sizes were determined via elution against a 100 bp DNA ladder. D–H, Photomicrographs of CaMKII ir (green) and, in red, HCN1 ir (D, E), HCN2 ir (F), HCN3 ir (G), and HCN4 ir (H) in the BLA (bregma, −2.56 mm). The arrows indicate CaMKII-immunoreactive cells that are closely associated with HCN expression; arrowheads indicate single labeled CaMKII- or HCN subunit-immunoreactive structures. Scale bars: D, F, G, H (all with 60× objective), E (with 100× objective), 40 μm.
Similar articles
- NPY Induces Stress Resilience via Downregulation of _I_h in Principal Neurons of Rat Basolateral Amygdala.
Silveira Villarroel H, Bompolaki M, Mackay JP, Miranda Tapia AP, Michaelson SD, Leitermann RJ, Marr RA, Urban JH, Colmers WF. Silveira Villarroel H, et al. J Neurosci. 2018 May 9;38(19):4505-4520. doi: 10.1523/JNEUROSCI.3528-17.2018. Epub 2018 Apr 12. J Neurosci. 2018. PMID: 29650696 Free PMC article. - Contribution of NPY Y5 Receptors to the Reversible Structural Remodeling of Basolateral Amygdala Dendrites in Male Rats Associated with NPY-Mediated Stress Resilience.
Michaelson SD, Miranda Tapia AP, McKinty A, Silveira Villarroel H, Mackay JP, Urban JH, Colmers WF. Michaelson SD, et al. J Neurosci. 2020 Apr 15;40(16):3231-3249. doi: 10.1523/JNEUROSCI.2621-19.2020. Epub 2020 Mar 6. J Neurosci. 2020. PMID: 32144180 Free PMC article. - The role of neuropeptide Y in the amygdala on corticotropin-releasing factor receptor-mediated behavioral stress responses in the rat.
Sajdyk TJ, Fitz SD, Shekhar A. Sajdyk TJ, et al. Stress. 2006 Mar;9(1):21-8. doi: 10.1080/10253890600557315. Stress. 2006. PMID: 16753930 - Interactions between NPY and CRF in the amygdala to regulate emotionality.
Sajdyk TJ, Shekhar A, Gehlert DR. Sajdyk TJ, et al. Neuropeptides. 2004 Aug;38(4):225-34. doi: 10.1016/j.npep.2004.05.006. Neuropeptides. 2004. PMID: 15337374 Review. - The central amygdala and alcohol: role of γ-aminobutyric acid, glutamate, and neuropeptides.
Roberto M, Gilpin NW, Siggins GR. Roberto M, et al. Cold Spring Harb Perspect Med. 2012 Dec 1;2(12):a012195. doi: 10.1101/cshperspect.a012195. Cold Spring Harb Perspect Med. 2012. PMID: 23085848 Free PMC article. Review.
Cited by
- Activation of neuropeptide S-expressing neurons in the locus coeruleus by corticotropin-releasing factor.
Jüngling K, Liu X, Lesting J, Coulon P, Sosulina L, Reinscheid RK, Pape HC. Jüngling K, et al. J Physiol. 2012 Aug 15;590(16):3701-17. doi: 10.1113/jphysiol.2011.226423. Epub 2012 May 8. J Physiol. 2012. PMID: 22570383 Free PMC article. - Ethanol withdrawal-induced adaptations in prefrontal corticotropin releasing factor receptor 1-expressing neurons regulate anxiety and conditioned rewarding effects of ethanol.
Patel RR, Wolfe SA, Borgonetti V, Gandhi PJ, Rodriguez L, Snyder AE, D'Ambrosio S, Bajo M, Domissy A, Head S, Contet C, Dayne Mayfield R, Roberts AJ, Roberto M. Patel RR, et al. Mol Psychiatry. 2022 Aug;27(8):3441-3451. doi: 10.1038/s41380-022-01642-3. Epub 2022 Jun 6. Mol Psychiatry. 2022. PMID: 35668157 Free PMC article. - Epigenetics of the depressed brain: role of histone acetylation and methylation.
Sun H, Kennedy PJ, Nestler EJ. Sun H, et al. Neuropsychopharmacology. 2013 Jan;38(1):124-37. doi: 10.1038/npp.2012.73. Epub 2012 Jun 13. Neuropsychopharmacology. 2013. PMID: 22692567 Free PMC article. Review. - Exploring the Therapeutic Effect of Neurotrophins and Neuropeptides in Neurodegenerative Diseases: at a Glance.
Rahman MM, Islam MR, Supti FA, Dhar PS, Shohag S, Ferdous J, Shuvo SK, Akter A, Hossain MS, Sharma R. Rahman MM, et al. Mol Neurobiol. 2023 Aug;60(8):4206-4231. doi: 10.1007/s12035-023-03328-5. Epub 2023 Apr 13. Mol Neurobiol. 2023. PMID: 37052791 Review.
References
- Accili EA, Proenza C, Baruscotti M, DiFrancesco D. From funny current to HCN channels: 20 years of excitation. News Physiol Sci. 2002;17:32–37. - PubMed
- Alonso J, Lépine JP ESEMeD/MHEDEA 2000 Scientific Committee. Overview of key data from the European Study of the Epidemiology of Mental Disorders (ESEMeD) J Clin Psychiatry. 2007;68(Suppl 2):3–9. - PubMed
- Amaral DG. The primate amygdala and the neurobiology of social behavior: implications for understanding social anxiety. Biol Psychiatry. 2002;51:11–17. - PubMed
- Berger T, Larkum ME, Lüscher HR. High Ih channel density in the distal apical dendrite of layer V pyramidal cells increases bidirectional attenuation of EPSPs. J Neurophysiol. 2001;85:855–868. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 MH081152/MH/NIMH NIH HHS/United States
- R01 MH062621/MH/NIMH NIH HHS/United States
- U24 NS050606/NS/NINDS NIH HHS/United States
- MH62121/MH/NIMH NIH HHS/United States
- MOP 10250/CAPMC/ CIHR/Canada
- MH081152/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous