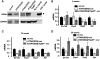Partial loss of Tip60 slows mid-stage neurodegeneration in a spinocerebellar ataxia type 1 (SCA1) mouse model - PubMed (original) (raw)
. 2011 Jun 1;20(11):2204-12.
doi: 10.1093/hmg/ddr108. Epub 2011 Mar 22.
Affiliations
- PMID: 21427130
- PMCID: PMC3090197
- DOI: 10.1093/hmg/ddr108
Partial loss of Tip60 slows mid-stage neurodegeneration in a spinocerebellar ataxia type 1 (SCA1) mouse model
Kristin M Gehrking et al. Hum Mol Genet. 2011.
Abstract
Spinocerebellar ataxia type 1 (SCA1) is one of nine dominantly inherited neurodegenerative diseases caused by polyglutamine tract expansion. In SCA1, the expanded polyglutamine tract is in the ataxin-1 (ATXN1) protein. ATXN1 is part of an in vivo complex with retinoid acid receptor-related orphan receptor alpha (Rora) and the acetyltransferase tat-interactive protein 60 kDa (Tip60). ATXN1 and Tip60 interact directly via the ATXN1 and HMG-box protein 1 (AXH) domain of ATXN1. Moreover, the phospho-mimicking Asp amino acid at position 776, previously shown to enhance pathogenesis, increases the ability of ATXN1 to interact with Tip60. Using a genetic approach, the biological relevance of the ATXN1/Tip60 interaction was assessed by crossing ATXN1[82Q] mice with Tip60(+/-)animals. Partial Tip60 loss increased Rora and Rora-mediated gene expression and delayed ATXN1[82]-mediated cerebellar degeneration during mid-stage disease progression. These results suggested a specific, temporal role for Tip60 during disease progression. We also showed that genetic background modulated ATXN1[82Q]-induced phenotypes. Of interest, these latter studies showed that some phenotypes are enhanced on a mixed background while others are suppressed.
Figures
Figure 1.
The interaction of ATXN1 with Tip60 requires the AXH domain of ATXN1 and is enhanced by a phospho-mimicking Asp at residue 776. (A) Diagrams of the GST–ATXN1 constructs used to pull-down His-tagged 35S-labeled Tip60. (B) Representative polyacrylamide gel showing the ability of GST–ATXN1 to pull-down His-tagged 35S-labeled Tip60. Upper panel depicts an autoradiograph of 35S-labeled Tip60 pulled down by each GST–ATXN1 construct. Lower panel shows Coomassie stained GST–ATXN1 constructs. (C) Quantification of Tip60 pulled down by GST–ATXN1–fragment V in which the amino acid at position 776 was either a Ser (S776), a phosphorylation resistant Ala (S776A) or phospho-mimicking Asp (S776D).
Figure 2.
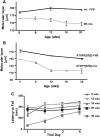
Genetic background affects SCA1 phenotypes in ATXN1[82Q] transgenic mice. (A) Cerebellar molecular layer thickness in aging WT-FVB and FVB;SV-129;C57BL/6 (mix) mice (n = 3–7 mice/genotype/age). (B) Cerebellar molecular layer thickness in aging FVB and FVB;SV-129;C57BL/6 (mix) mice expressing the ATXN1[82Q] transgene (n = 3–7 mice/genotype/age). (C) Motor performance using the accelerating rotarod paradigm in aging FVB;SV-129;C57BL/6 (mix) mice expressing the ATXN1[82Q] transgene (n = 6–12 mice/genotype).
Figure 3.
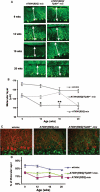
Tip60 haploinsufficiency rescues SCA1 cerebellar pathology during the mid-stage of disease. (A) Calbindin immunofluorescence of Purkinje cells in aging WT, ATXN1[82Q] and ATXN1[82Q]:Tip60+/− FVB;SV-129;C57BL/6 (mix) mice. (B) Quantitative analysis of the molecular thickness in aging WT, ATXN1[82Q] and ATXN1[82Q]:Tip60+/− FVB;SV-129;C57BL/6 (mix) mice (n = 3–7 mice/genotype/age). (C) Immunofluorescence of calbindin (red) and VGluT2 (green) in WT, ATXN1[82Q] and ATXN1[82Q]:Tip60+/− FVB;SV-129;C57BL/6 (mix) mice. (D) Quantitative analysis of the climbing fiber extension along Purkinje cell dendrites in aging WT, ATXN1[82Q] and ATXN1[82Q]:Tip60+/− FVB;SV-129;C57BL/6 (mix) mice (n = 3–5 mice/genotype/age).
Figure 4.
Tip60 haploinsufficiency restoration of Rora levels (n = 3 mice) and Rora-mediated gene expression. (A) Western blot analysis of cerebellar Rora levels in WT, ATXN1[82Q] and ATXN1[82Q]:Tip60+/− FVB;SV-129;C57BL/6 (mix) mice, and in homozygous staggerer (sg/sg) mice at 12 weeks of age. COS cells transfected with Rora cDNA are shown as a positive control. (B) Quantitative PCR of four Rora-mediated genes in WT, ATXN1[82Q] and ATXN1[82Q]:Tip60+/− FVB;SV-129;C57BL/6 (mix) mice at 8 weeks of age. (C) Quantitative PCR of four Rora-mediated genes in WT, ATXN1[82Q] and ATXN1[82Q]Tip60+/− FVB;SV-129;C57BL/6 (mix) mice at 12 weeks of age. (D) Quantitative PCR of four Rora-mediated genes in WT, ATXN1[82Q] and ATXN1[82Q]:Tip60+/− FVB;SV-129;C57BL/6 (mix) mice at 20 weeks of age.
Similar articles
- Partial loss of ataxin-1 function contributes to transcriptional dysregulation in spinocerebellar ataxia type 1 pathogenesis.
Crespo-Barreto J, Fryer JD, Shaw CA, Orr HT, Zoghbi HY. Crespo-Barreto J, et al. PLoS Genet. 2010 Jul 8;6(7):e1001021. doi: 10.1371/journal.pgen.1001021. PLoS Genet. 2010. PMID: 20628574 Free PMC article. - Serine 776 of ataxin-1 is critical for polyglutamine-induced disease in SCA1 transgenic mice.
Emamian ES, Kaytor MD, Duvick LA, Zu T, Tousey SK, Zoghbi HY, Clark HB, Orr HT. Emamian ES, et al. Neuron. 2003 May 8;38(3):375-87. doi: 10.1016/s0896-6273(03)00258-7. Neuron. 2003. PMID: 12741986 - Purkinje cell ataxin-1 modulates climbing fiber synaptic input in developing and adult mouse cerebellum.
Ebner BA, Ingram MA, Barnes JA, Duvick LA, Frisch JL, Clark HB, Zoghbi HY, Ebner TJ, Orr HT. Ebner BA, et al. J Neurosci. 2013 Mar 27;33(13):5806-20. doi: 10.1523/JNEUROSCI.6311-11.2013. J Neurosci. 2013. PMID: 23536093 Free PMC article. - Beyond the glutamine expansion: influence of posttranslational modifications of ataxin-1 in the pathogenesis of spinocerebellar ataxia type 1.
Ju H, Kokubu H, Lim J. Ju H, et al. Mol Neurobiol. 2014 Dec;50(3):866-874. doi: 10.1007/s12035-014-8703-z. Epub 2014 Apr 22. Mol Neurobiol. 2014. PMID: 24752589 Free PMC article. Review. - Pathogenic mechanisms of a polyglutamine-mediated neurodegenerative disease, spinocerebellar ataxia type 1.
Zoghbi HY, Orr HT. Zoghbi HY, et al. J Biol Chem. 2009 Mar 20;284(12):7425-9. doi: 10.1074/jbc.R800041200. Epub 2008 Oct 28. J Biol Chem. 2009. PMID: 18957430 Free PMC article. Review.
Cited by
- Longitudinal single-cell transcriptional dynamics throughout neurodegeneration in SCA1.
Tejwani L, Ravindra NG, Lee C, Cheng Y, Nguyen B, Luttik K, Ni L, Zhang S, Morrison LM, Gionco J, Xiang Y, Yoon J, Ro H, Haidery F, Grijalva RM, Bae E, Kim K, Martuscello RT, Orr HT, Zoghbi HY, McLoughlin HS, Ranum LPW, Shakkottai VG, Faust PL, Wang S, van Dijk D, Lim J. Tejwani L, et al. Neuron. 2024 Feb 7;112(3):362-383.e15. doi: 10.1016/j.neuron.2023.10.039. Epub 2023 Nov 27. Neuron. 2024. PMID: 38016472 Free PMC article. - Integration of modeling with experimental and clinical findings synthesizes and refines the central role of inositol 1,4,5-trisphosphate receptor 1 in spinocerebellar ataxia.
Brown SA, Loew LM. Brown SA, et al. Front Neurosci. 2015 Jan 21;8:453. doi: 10.3389/fnins.2014.00453. eCollection 2014. Front Neurosci. 2015. PMID: 25653583 Free PMC article. Review. - Polyglutamine (polyQ) disorders: the chromatin connection.
Cohen-Carmon D, Meshorer E. Cohen-Carmon D, et al. Nucleus. 2012 Sep-Oct;3(5):433-41. doi: 10.4161/nucl.21481. Epub 2012 Aug 15. Nucleus. 2012. PMID: 22892726 Free PMC article. Review. - Gene Deregulation and Underlying Mechanisms in Spinocerebellar Ataxias With Polyglutamine Expansion.
Niewiadomska-Cimicka A, Hache A, Trottier Y. Niewiadomska-Cimicka A, et al. Front Neurosci. 2020 Jun 9;14:571. doi: 10.3389/fnins.2020.00571. eCollection 2020. Front Neurosci. 2020. PMID: 32581696 Free PMC article. Review. - SCA1-phosphorylation, a regulator of Ataxin-1 function and pathogenesis.
Orr HT. Orr HT. Prog Neurobiol. 2012 Dec;99(3):179-85. doi: 10.1016/j.pneurobio.2012.04.003. Epub 2012 Apr 16. Prog Neurobiol. 2012. PMID: 22531670 Free PMC article. Review.
References
- Orr H.T., Chung M.Y., Banfi S., Kwiatkowski T.J., Jr, Servadio A., Beaudet A.L., McCall A.E., Duvick L.A., Ranum L.P., Zoghbi H.Y. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat. Genet. 1993;4:221–226. doi:10.1038/ng0793-221. - DOI - PubMed
- Orr H.T., Zoghbi H.Y. Trinucleotide repeat disorders. Ann. Rev. Neurosci. 2007;30:575–621. doi:10.1146/annurev.neuro.29.051605.113042. - DOI - PubMed
- Servadio A., Koshy B., Armstrong D., Antalffy B., Orr H.T., Zoghbi H.Y. Expression analysis of the ataxin-1 protein in tissues from normal and spinocerebellar ataxia type 1 individuals. Nat. Genet. 1995;10:94–98. doi:10.1038/ng0595-94. - DOI - PubMed
- Banfi S., Servadio A., Chung M., Capozzoli F., Duvick L.A., Elde R., Zoghbi H.Y., Orr H.T. Cloning and developmental expression analysis of the murine homolog of the spinocerebellar ataxia type 1 gene (Sca1) Hum. Mol. Genet. 1996;5:33–40. doi:10.1093/hmg/5.1.33. - DOI - PubMed
- Koeppen A.H. The pathogenesis of spinocerebellar ataxia. Cerebellum. 2005;4:62–73. doi:10.1080/14734220510007950. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous