Targeting SDF-1/CXCR4 to inhibit tumour vasculature for treatment of glioblastomas - PubMed (original) (raw)
Review
. 2011 Jun 7;104(12):1805-9.
doi: 10.1038/bjc.2011.169. Epub 2011 May 17.
Affiliations
- PMID: 21587260
- PMCID: PMC3111201
- DOI: 10.1038/bjc.2011.169
Review
Targeting SDF-1/CXCR4 to inhibit tumour vasculature for treatment of glioblastomas
D Tseng et al. Br J Cancer. 2011.
Abstract
Local recurrence of glioblastomas is a major cause of patient mortality after definitive treatment. This review discusses the roles of the chemokine stromal cell-derived factor-1 and its receptor CXC chemokine receptor 4 (CXCR4) in affecting the sensitivity of glioblastomas to irradiation. Blocking these molecules prevents or delays tumour recurrence after irradiation by inhibiting the recruitment of CD11b+ monocytes/macrophages that participate in revascularising the tumour. We review the literature pertaining to the mechanism by which revascularisation occurs following tumour irradiation using experimental models. Areas of interest and debate in the literature include the process by which endothelial cells die after irradiation and the identity/origin of the cells that reconstitute the tumour blood vessels after injury. Understanding the processes that mediate tumour revascularisation will guide the improvement of clinical strategies for preventing recurrence of glioblastoma after irradiation.
Figures
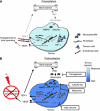
Figure 1
Model of the main contributions of bone marrow-derived cells (BMDCs) and cytokines that promote restoration of tumour vasculature following irradiation. Prior to irradiation, tumour growth is governed largely by local angiogenesis. When local angiogenesis is inhibited by irradiation, growth of the tumour vasculature (essential for recurrence of the tumour) can only occur from circulating cells, of which BMDCs are an essential component. Following irradiation, the tumour becomes more hypoxic and HIF-1 is increased as the tumour attempts to regrow. This induces SDF-1 and promotes the recruitment of CD11b+ monocytes/macrophages and retention of these cells in the tumour. Stromal cell-derived factor-1/CXC chemokine receptor 4 is the key interaction for the influx of BMDCs as AMD3100, an inhibitor of CXCR4/SDF-1, and antibodies against CXCR4 block the recruitment and/or tumour retention of the BMDCs, inhibit restoration of the tumour vasculature, and prevent tumour recurrence. The various inhibitors and the points in the cycle at which they act are shown in boxes. Reproduced from Kioi et al (2010) with permission. (A) Pre-irradiation; (B) post-irradiation.
Similar articles
- Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice.
Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Kioi M, et al. J Clin Invest. 2010 Mar;120(3):694-705. doi: 10.1172/JCI40283. Epub 2010 Feb 22. J Clin Invest. 2010. PMID: 20179352 Free PMC article. - Blockade of SDF-1 after irradiation inhibits tumor recurrences of autochthonous brain tumors in rats.
Liu SC, Alomran R, Chernikova SB, Lartey F, Stafford J, Jang T, Merchant M, Zboralski D, Zöllner S, Kruschinski A, Klussmann S, Recht L, Brown JM. Liu SC, et al. Neuro Oncol. 2014 Jan;16(1):21-8. doi: 10.1093/neuonc/not149. Epub 2013 Dec 10. Neuro Oncol. 2014. PMID: 24335554 Free PMC article. - Vasculogenesis: a crucial player in the resistance of solid tumours to radiotherapy.
Brown JM. Brown JM. Br J Radiol. 2014 Mar;87(1035):20130686. doi: 10.1259/bjr.20130686. Br J Radiol. 2014. PMID: 24338942 Free PMC article. Review. - Recurrence of glioblastoma after radio-chemotherapy is associated with an angiogenic switch to the CXCL12-CXCR4 pathway.
Tabouret E, Tchoghandjian A, Denicolai E, Delfino C, Metellus P, Graillon T, Boucard C, Nanni I, Padovani L, Ouafik L, Figarella-Branger D, Chinot O. Tabouret E, et al. Oncotarget. 2015 May 10;6(13):11664-75. doi: 10.18632/oncotarget.3256. Oncotarget. 2015. PMID: 25860928 Free PMC article. - The CXCR4/SDF-1 chemokine axis: a potential therapeutic target for bone metastases?
Hirbe AC, Morgan EA, Weilbaecher KN. Hirbe AC, et al. Curr Pharm Des. 2010;16(11):1284-90. doi: 10.2174/138161210791034012. Curr Pharm Des. 2010. PMID: 20166978 Review.
Cited by
- Role of bone marrow-derived cells in angiogenesis: focus on macrophages and pericytes.
Ding Y, Song N, Luo Y. Ding Y, et al. Cancer Microenviron. 2012 Dec;5(3):225-36. doi: 10.1007/s12307-012-0106-y. Epub 2012 Apr 20. Cancer Microenviron. 2012. PMID: 22528877 Free PMC article. - PIEZO1 Promotes the Migration of Endothelial Cells via Enhancing CXCR4 Expression under Simulated Microgravity.
Wang Y, Li C, Wang R, Zhao X, Pan Y, Zhang Q, Li S, Fan J, Wang Y, Sun X. Wang Y, et al. Int J Mol Sci. 2024 Jul 1;25(13):7254. doi: 10.3390/ijms25137254. Int J Mol Sci. 2024. PMID: 39000362 Free PMC article. - Fluorinated PEG-PEI Coated Magnetic Nanoparticles for siRNA Delivery and CXCR4 Knockdown.
Cao Y, Zhang S, Ma M, Zhang Y. Cao Y, et al. Nanomaterials (Basel). 2022 May 16;12(10):1692. doi: 10.3390/nano12101692. Nanomaterials (Basel). 2022. PMID: 35630915 Free PMC article. - Metastasis review: from bench to bedside.
Alizadeh AM, Shiri S, Farsinejad S. Alizadeh AM, et al. Tumour Biol. 2014 Sep;35(9):8483-523. doi: 10.1007/s13277-014-2421-z. Epub 2014 Aug 8. Tumour Biol. 2014. PMID: 25104089 Review. - Macrophage polarization by MSC-derived CXCL12 determines tumor growth.
Babazadeh S, Nassiri SM, Siavashi V, Sahlabadi M, Hajinasrollah M, Zamani-Ahmadmahmudi M. Babazadeh S, et al. Cell Mol Biol Lett. 2021 Jun 26;26(1):30. doi: 10.1186/s11658-021-00273-w. Cell Mol Biol Lett. 2021. PMID: 34174813 Free PMC article.
References
- Aghi M, Cohen KS, Klein RJ, Scadden DT, Chiocca EA (2006) Tumor stromal-derived factor-1 recruits vascular progenitors to mitotic neovasculature, where microenvironment influences their differentiated phenotypes. Cancer Res 66(18): 9054–9064 - PubMed
- Aicher A, Rentsch M, Sasaki K, Ellwart JW, Fandrich F, Siebert R, Cooke JP, Dimmeler S, Heeschen C (2007) Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ Res 100(4): 581–589 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials