Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance - PubMed (original) (raw)
Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance
Dawang Zhou et al. Proc Natl Acad Sci U S A. 2011.
Abstract
Ablation of the kinases Mst1 and Mst2, orthologs of the Drosophila antiproliferative kinase Hippo, from mouse intestinal epithelium caused marked expansion of an undifferentiated stem cell compartment and loss of secretory cells throughout the small and large intestine. Although median survival of mice lacking intestinal Mst1/Mst2 is 13 wk, adenomas of the distal colon are common by this age. Diminished phosphorylation, enhanced abundance, and nuclear localization of the transcriptional coactivator Yes-associated protein 1 (Yap1) is evident in Mst1/Mst2-deficient intestinal epithelium, as is strong activation of β-catenin and Notch signaling. Although biallelic deletion of Yap1 from intestinal epithelium has little effect on intestinal development, inactivation of a single Yap1 allele reduces Yap1 polypeptide abundance to nearly wild-type levels and, despite the continued Yap hypophosphorylation and preferential nuclear localization, normalizes epithelial structure. Thus, supraphysiologic Yap polypeptide levels are necessary to drive intestinal stem cell proliferation. Yap is overexpressed in 68 of 71 human colon cancers and in at least 30 of 36 colon cancer-derived cell lines. In colon-derived cell lines where Yap is overabundant, its depletion strongly reduces β-catenin and Notch signaling and inhibits proliferation and survival. These findings demonstrate that Mst1 and Mst2 actively suppress Yap1 abundance and action in normal intestinal epithelium, an antiproliferative function that frequently is overcome in colon cancer through Yap1 polypeptide overabundance. The dispensability of Yap1 in normal intestinal homeostasis and its potent proliferative and prosurvival actions when overexpressed in colon cancer make it an attractive therapeutic target.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Mst1null/Mst2ff/villin-Cre mice exhibit early mortality and impaired epithelial differentiation in the small intestine. (A) A Kaplan–Meier survival curve from eight wild-type and 12 Mst1null/Mst2ff/villin-Cre (double-knockout, dko) mice, the latter with a average median survival of 13 wk. (B) Body weight of Mst1null/Mst2ff/villin-Cre (dko) and wild-type mice at age 6 wk. (C) Representative examples of intestinal tract from Mst1null/Mst2ff/villin-Cre (DKO) and wild-type mice at age 6 wk (n = 3 per group). Ce, cecum; s, stomach. (D_–_H) Representative samples showing absence of secretory cell lineages in the small intestine of Mst1null/Mst2ff/villin-Cre mice (DKO) compared with wild-type mice (n = 3 per group).
Fig. 2.
Mst1null/Mst2ff/villin-Cre (DKO) mice exhibit loss of goblet cells, epithelial dysplasia, and adenomas in the colon. (A) Hyperproliferation and dysplasia of the colonic epithelium in 6-wk-old Mst1null/Mst2ff/villin-Cre (DKO) mice. (B) Loss of goblet cells in proximal and distal colon of 6-wk-old Mst1null/Mst2ff/villin-Cre mice. Tissues shown are representative of three mutant and three wild-type mice. Boxed areas in low-magnification (L) views are shown to the right at higher magnification (H). Histologic views of the cecum are shown in
SI Appendix, Fig. S1
. (C) Formation of epithelial hyperplasia, dysplasia, and adenoma (arrows, Upper right) in the distal colon in two Mst1null/Mst2ff/villin-Cre mice, age 15 wk (Upper) and 20 wk (Lower). (D) Expression of the Mst1, Mst2, and Yap1 polypeptides in the mouse intestinal tract. C, colon; c, cecum; d, duodenum; i, ileum, j, jejunum; SI, small intestine.
Fig. 3.
Expression of Mst1, Mst2, Yap, and Wnt targets in the intestine of Mst1null/Mst2ff/villin-Cre and wild-type mice. (A) Western blot of mucosal epithelium from the indicated intestinal segments of 6-wk-old Mst1null/Mst2ff/villin-Cre and wild-type mice for Mst1, Mst2 Yap, Yap(Ser127P), and the Wnt target polypeptides as indicated. ABC, activated β-catenin. SI, small intestine. (B) Quantitation of the number of Yap1+ and Ki67+ cells detected by immunofluorescence staining of small intestine (SI) and colon of Mst1null/Mst2ff/villin-Cre and wild-type mice. These results represent analyses of three mice of each genotype ± SE. (n = 20 crypts per section, 3 mice of each genotype; **P < 0.01 and ***P < 0.001 vs. WT). A representative immunofluorescent image of Yap1, Ki67, and β-catenin staining is shown in
SI Appendix, Fig. S3
. (C) SW480 cells stably expressing lentiviral-encoded, doxycycline-inducible scrambled (s) or either of two Yap1-directed shRNAs (1 and 2) were treated with doxycycline or carrier at time 0, transfected with plasmids encoding either TOPflash or FOPflash and Renilla luciferase 24 h thereafter, and were extracted 48 h after addition of doxycycline or carrier. Luciferase activities were assayed. Firefly luciferase activity normalized for Renilla luciferase is shown. (D) SW480 cells as in Fig. 4_C_ expressing scrambled (s) or Yap-directed shRNA (1) were harvested 48 h after addition of doxycycline or carrier, and cytosolic and nuclear fractions were immunoblotted for Yap, β-catenin, actin, and histone H3.
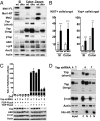
Fig. 4.
Notch signaling in the intestine of Mst1null/Mst2ff/villin-Cre and wild-type mice and in Yap-depleted HCT116 cells. (A) Western blot of mucosal epithelium from the indicated intestinal segments of 6-wk-old Mst1null/Mst2ff/villin-Cre and wild-type mice for Notch intracellular domain (NICD), the Notch targets Hes1 and Math1, Stat3, and PY-Stat3. SI, small intestine. (B) Immunofluorescence staining small intestine and colon of Mst1null/Mst2ff/villin-Cre and wild-type mice for Notch intracellular domain, Hes1, and Math1, each with DAPI. The areas in the dashed boxes are shown at higher magnification in Insets. Results in A and B are representative of three mice of each genotype. (C) HCT116 cells were engineered to express the following tetracycline-inducible shRNAs stably: a scrambled shRNA (s), one of three shRNAs each directed at a different segment of the Yap mRNA [Yap-sh1 (1); Yap-sh2 (2); or Yap-sh3 (3)], or all three shRNAs together (all). Extracts were subjected to immunoblot for the polypeptides shown 24 h after addition of doxycycline (+) or carrier (−).
Fig. 5.
The effect of deleting one or both Yap alleles on the morphology of the Mst1/Mst2-deficient intestinal epithelium. (A) A Kaplan–Meier plot comparing the survival of wild-type, Mst1null/Mst2ff/Yapf+/villin-Cre (dkoHet), and Mst1null/Mst2ff/Yapff/villin-Cre (tko) mice. Small intestine and colonic mucosa scrapings from these and from Mst1null/Mst2ff/villin-Cre and from Yapff/villin-Cre (Yapko) mice were extracted and immunoblotted for Yap. (B) Sections of small intestine (SI) (ileum) and colon from wild-type (Top), Mst1null/Mst2ff/Yapf+/villin-Cre (Middle), and Mst1null/Mst2ff/Yapff/villin-Cre (Bottom) mice were stained for lysozyme-, chromogranin-, or periodic acid Schiff (PAS)-positive material. Quantitation of the number of cells positive for these markers is shown in the bar graphs; data are ± SE. Open bars represent WT mice; light gray bars represent Mst1/Mst2ff/villin-Cre mice; dark gray bars represent Mst1null/Mst2ff/Yapf+/villin-Cre mice; black bars represent Mst1null/Mst2ff/Yapff/villin-Cre mice (n = 20 crypts per section, 3 mice of each genotype; ***P < 0.001 vs. WT). (C) Section of colon from wild-type and Mst1null/Mst2ff/Yapff/villin-Cre (TKO) mice stained for Yap with or without Ki67. The TKO panels in the upper row on the far right are from segments in which villin-Cre excision failed to occur, enabling a side-by side comparison of epithelia that are Mst1null but Mst2ff and Yap sufficient with epithelia that lack Mst1, Mst2, and Yap1; these samples are morphologically indistinguishable. The bottom rows show that the abundance of Ki67+ cells is similar in Mst-null and in Mst1null/Mst2ff/Yapff villin-Cre epithelium. (D) Extracts of normal mouse liver and colonic mucosa, and from Mst1null/Mst2ff/albumin-Cre mouse liver and from Mst1null/Mst2ff/villin-Cre colonic mucosa were immunoblotted for Yap and for the Yap phosphorylation sites indicated. The intensity of the Yap polypeptide, normalized for actin intensity, was divided into the value for each of the Yap-P signals; the relative phosphorylation at the two sites is shown in the bar graph with values for WT liver and colon set to 1.
Fig. 6.
The expression of Yap in human colon cancer tissue and cell lines and the effect of Yap shRNA on the proliferation of colon cancer-derived cell lines. (A) Yap immunohistochemistry of colon cancer tissue microarrays. (Right) A section of normal colon shows an occasional Yap+ cell in the crypt. Yap+ cells also are visualized in the lamina propria. Two cores were obtained from different regions of 71 colonic cancers; histologic sections were examined for the intensity and subcellular localization of Yap staining and scored as in described in Materials and Methods. (Left) The averaged score of the two samples from each tumor is shown in the table. Examples of the intratumoral heterogeneity of Yap staining are shown in
SI Appendix, Fig. S5_A_
). (B) Western blotting analysis of YAP expression and Yap(Ser127) phosphorylation in human colon cancer cell lines and mucosal epithelium isolated from 10 normal colons. (C) A qualitative estimate of the relative abundance of Yap polypeptide and Yap(Ser127) immunoreactivity (p-Yap1) in selected colon cancer cell lines and the extent of inhibition of colony formation of these lines after infection with lentiviruses encoding two different Yap shRNAs. Data shown are the average of two experiments). The primary data are shown in
SI Appendix, Fig. S7
.
Fig. P1.
Operation of the mammalian Hippo pathway in mouse intestinal epithelium and human colon cancer. (A) Normal mouse intestinal epithelium. (B) Absence of Mst1 and Mst2. (C) Inactivation of a single copy of the Yap1 gene in the Mst1/Mst2-deficient epithelium. (D) Inappropriate activation of β-catenin in human colon cancer.
Similar articles
- NDR functions as a physiological YAP1 kinase in the intestinal epithelium.
Zhang L, Tang F, Terracciano L, Hynx D, Kohler R, Bichet S, Hess D, Cron P, Hemmings BA, Hergovich A, Schmitz-Rohmer D. Zhang L, et al. Curr Biol. 2015 Feb 2;25(3):296-305. doi: 10.1016/j.cub.2014.11.054. Epub 2015 Jan 15. Curr Biol. 2015. PMID: 25601544 Free PMC article. - Hippo/Yap signaling controls epithelial progenitor cell proliferation and differentiation in the embryonic and adult lung.
Lange AW, Sridharan A, Xu Y, Stripp BR, Perl AK, Whitsett JA. Lange AW, et al. J Mol Cell Biol. 2015 Feb;7(1):35-47. doi: 10.1093/jmcb/mju046. Epub 2014 Dec 5. J Mol Cell Biol. 2015. PMID: 25480985 Free PMC article. - Tubule-Specific Mst1/2 Deficiency Induces CKD via YAP and Non-YAP Mechanisms.
Xu C, Wang L, Zhang Y, Li W, Li J, Wang Y, Meng C, Qin J, Zheng ZH, Lan HY, Mak KK, Huang Y, Xia Y. Xu C, et al. J Am Soc Nephrol. 2020 May;31(5):946-961. doi: 10.1681/ASN.2019101052. Epub 2020 Apr 6. J Am Soc Nephrol. 2020. PMID: 32253273 Free PMC article. - YAP oncogene overexpression supercharges colon cancer proliferation.
Avruch J, Zhou D, Bardeesy N. Avruch J, et al. Cell Cycle. 2012 Mar 15;11(6):1090-6. doi: 10.4161/cc.11.6.19453. Epub 2012 Mar 15. Cell Cycle. 2012. PMID: 22356765 Free PMC article. Review. - Hippo pathway regulation of gastrointestinal tissues.
Yu FX, Meng Z, Plouffe SW, Guan KL. Yu FX, et al. Annu Rev Physiol. 2015;77:201-27. doi: 10.1146/annurev-physiol-021014-071733. Epub 2014 Sep 29. Annu Rev Physiol. 2015. PMID: 25293527 Review.
Cited by
- Hippo-Foxa2 signaling pathway plays a role in peripheral lung maturation and surfactant homeostasis.
Chung C, Kim T, Kim M, Kim M, Song H, Kim TS, Seo E, Lee SH, Kim H, Kim SK, Yoo G, Lee DH, Hwang DS, Kinashi T, Kim JM, Lim DS. Chung C, et al. Proc Natl Acad Sci U S A. 2013 May 7;110(19):7732-7. doi: 10.1073/pnas.1220603110. Epub 2013 Apr 25. Proc Natl Acad Sci U S A. 2013. PMID: 23620511 Free PMC article. - Signal integration in TGF-β, WNT, and Hippo pathways.
Attisano L, Wrana JL. Attisano L, et al. F1000Prime Rep. 2013 Jun 3;5:17. doi: 10.12703/P5-17. Print 2013. F1000Prime Rep. 2013. PMID: 23755364 Free PMC article. - Hippo signalling directs intestinal fate.
Le Bouteiller M, Jensen KB. Le Bouteiller M, et al. Nat Cell Biol. 2015 Jan;17(1):5-6. doi: 10.1038/ncb3086. Nat Cell Biol. 2015. PMID: 25679030 - The Hippo signaling pathway and stem cell biology.
Ramos A, Camargo FD. Ramos A, et al. Trends Cell Biol. 2012 Jul;22(7):339-46. doi: 10.1016/j.tcb.2012.04.006. Epub 2012 May 31. Trends Cell Biol. 2012. PMID: 22658639 Free PMC article. Review. - NDR functions as a physiological YAP1 kinase in the intestinal epithelium.
Zhang L, Tang F, Terracciano L, Hynx D, Kohler R, Bichet S, Hess D, Cron P, Hemmings BA, Hergovich A, Schmitz-Rohmer D. Zhang L, et al. Curr Biol. 2015 Feb 2;25(3):296-305. doi: 10.1016/j.cub.2014.11.054. Epub 2015 Jan 15. Curr Biol. 2015. PMID: 25601544 Free PMC article.
References
- Dan I, Watanabe NM, Kusumi A. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 2001;11:220–230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 DK007028/DK/NIDDK NIH HHS/United States
- R01 DK17776/DK/NIDDK NIH HHS/United States
- R01 CA136567/CA/NCI NIH HHS/United States
- R37 DK017776/DK/NIDDK NIH HHS/United States
- R01 CA127306/CA/NCI NIH HHS/United States
- P30 DK040561/DK/NIDDK NIH HHS/United States
- CA136567/CA/NCI NIH HHS/United States
- CA127306/CA/NCI NIH HHS/United States
- P30 DK043351/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous