A pilot study of MUC-1/CEA/TRICOM poxviral-based vaccine in patients with metastatic breast and ovarian cancer - PubMed (original) (raw)
. 2011 Nov 15;17(22):7164-73.
doi: 10.1158/1078-0432.CCR-11-0649. Epub 2011 Nov 8.
Kwong-Yok Tsang, Ravi A Madan, Ngar-Yee Huen, Diane J Poole, Caroline Jochems, Jacquin Jones, Theresa Ferrara, Christopher R Heery, Philip M Arlen, Seth M Steinberg, Mary Pazdur, Myrna Rauckhorst, Elizabeth C Jones, William L Dahut, Jeffrey Schlom, James L Gulley
Affiliations
- PMID: 22068656
- PMCID: PMC3227395
- DOI: 10.1158/1078-0432.CCR-11-0649
A pilot study of MUC-1/CEA/TRICOM poxviral-based vaccine in patients with metastatic breast and ovarian cancer
Mahsa Mohebtash et al. Clin Cancer Res. 2011.
Abstract
Purpose: PANVAC is a recombinant poxviral vaccine that contains transgenes for MUC-1, CEA, and 3 T-cell costimulatory molecules. This study was conducted to obtain preliminary evidence of clinical response in metastatic breast and ovarian cancer patients.
Experimental design: Twenty-six patients were enrolled and given monthly vaccinations. Clinical and immune outcomes were evaluated.
Results: These patients were heavily pretreated, with 21 of 26 patients having 3 or more prior chemotherapy regimens. Side effects were largely limited to mild injection-site reactions. For the 12 breast cancer patients enrolled, median time to progression was 2.5 months (1-37+) and median overall survival was 13.7 months. Four patients had stable disease. One patient had a complete response by RECIST and remained on study for 37 months or more, with a significant drop in serum interleukin (IL)-6 and IL-8 by day 71. Another patient with metastatic disease confined to the mediastinum had a 17% reduction in mediastinal mass and was on study for 10 months. Patients with stable or responding disease had fewer prior therapies and lower tumor marker levels than patients with no evidence of response. For the ovarian cancer patients (n = 14), the median time to progression was 2 months (1-6) and median overall survival was 15.0 months. Updated data are presented here for one patient treated with this vaccine in a previous trial, with a time to progression of 38 months.
Conclusions: Some patients who had limited tumor burden with minimal prior chemotherapy seemed to benefit from the vaccine. Further studies to confirm these results are warranted.
Figures
Fig. 1
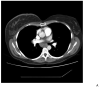
Patient 24 had a subcarinal lymph node mass measuring 2.8 cm (panel A) which decreased to 0.8 cm (panel B) after 10 months of vaccine, a > 50% reduction by RECIST, followed by a CR after 18 months (panel C). She remains on study 37 months after enrollment, with a durable CR.
Fig. 1
Patient 24 had a subcarinal lymph node mass measuring 2.8 cm (panel A) which decreased to 0.8 cm (panel B) after 10 months of vaccine, a > 50% reduction by RECIST, followed by a CR after 18 months (panel C). She remains on study 37 months after enrollment, with a durable CR.
Fig. 1
Patient 24 had a subcarinal lymph node mass measuring 2.8 cm (panel A) which decreased to 0.8 cm (panel B) after 10 months of vaccine, a > 50% reduction by RECIST, followed by a CR after 18 months (panel C). She remains on study 37 months after enrollment, with a durable CR.
Comment in
- Outlining novel scenarios for improved therapeutic cancer vaccines: the PANVAC paradigm.
Baxevanis CN. Baxevanis CN. Expert Rev Vaccines. 2012 Mar;11(3):275-7. doi: 10.1586/erv.11.193. Expert Rev Vaccines. 2012. PMID: 22380820
Similar articles
- Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma.
Gulley JL, Arlen PM, Tsang KY, Yokokawa J, Palena C, Poole DJ, Remondo C, Cereda V, Jones JL, Pazdur MP, Higgins JP, Hodge JW, Steinberg SM, Kotz H, Dahut WL, Schlom J. Gulley JL, et al. Clin Cancer Res. 2008 May 15;14(10):3060-9. doi: 10.1158/1078-0432.CCR-08-0126. Clin Cancer Res. 2008. PMID: 18483372 Free PMC article. Clinical Trial. - Vaccine therapy of established tumors in the absence of autoimmunity.
Hodge JW, Grosenbach DW, Aarts WM, Poole DJ, Schlom J. Hodge JW, et al. Clin Cancer Res. 2003 May;9(5):1837-49. Clin Cancer Res. 2003. PMID: 12738742 - Poxvirus-based vaccine therapy for patients with advanced pancreatic cancer.
Kaufman HL, Kim-Schulze S, Manson K, DeRaffele G, Mitcham J, Seo KS, Kim DW, Marshall J. Kaufman HL, et al. J Transl Med. 2007 Nov 26;5:60. doi: 10.1186/1479-5876-5-60. J Transl Med. 2007. PMID: 18039393 Free PMC article. Clinical Trial. - PANVAC-VF: poxviral-based vaccine therapy targeting CEA and MUC1 in carcinoma.
Madan RA, Arlen PM, Gulley JL. Madan RA, et al. Expert Opin Biol Ther. 2007 Apr;7(4):543-54. doi: 10.1517/14712598.7.4.543. Expert Opin Biol Ther. 2007. PMID: 17373905 Review. - Clinical evaluation of TRICOM vector therapeutic cancer vaccines.
Madan RA, Bilusic M, Heery C, Schlom J, Gulley JL. Madan RA, et al. Semin Oncol. 2012 Jun;39(3):296-304. doi: 10.1053/j.seminoncol.2012.02.010. Semin Oncol. 2012. PMID: 22595052 Free PMC article. Review.
Cited by
- Advances in predicting breast cancer driver mutations: Tools for precision oncology (Review).
Hao W, Rajendran BK, Cui T, Sun J, Zhao Y, Palaniyandi T, Selvam M. Hao W, et al. Int J Mol Med. 2025 Jan;55(1):6. doi: 10.3892/ijmm.2024.5447. Epub 2024 Oct 25. Int J Mol Med. 2025. PMID: 39450552 Free PMC article. Review. - Present and Future of Immunotherapy for Triple-Negative Breast Cancer.
Sriramulu S, Thoidingjam S, Speers C, Nyati S. Sriramulu S, et al. Cancers (Basel). 2024 Sep 24;16(19):3250. doi: 10.3390/cancers16193250. Cancers (Basel). 2024. PMID: 39409871 Free PMC article. Review. - Paeoniflorigenone inhibits ovarian cancer metastasis through targeting the MUC1/Wnt/β‑catenin pathway.
Liu Q, Jiang L, Zhao Y, Su F, Li J, Tian X, Liu W, Jiang X, Xu Y, Tao F. Liu Q, et al. Int J Mol Med. 2024 Jul;54(1):60. doi: 10.3892/ijmm.2024.5384. Epub 2024 May 24. Int J Mol Med. 2024. PMID: 38785138 Free PMC article. - Oncolytic viruses: A novel treatment strategy for breast cancer.
Javanbakht M, Tahmasebzadeh S, Cegolon L, Gholami N, Kashaki M, Nikoueinejad H, Mozafari M, Mozaffari M, Zhao S, Khafaei M, Izadi M, Fathi S, Akhavan-Sigari R. Javanbakht M, et al. Genes Dis. 2021 Dec 16;10(2):430-446. doi: 10.1016/j.gendis.2021.11.011. eCollection 2023 Mar. Genes Dis. 2021. PMID: 37223527 Free PMC article. Review. - Viral Vectors in Gene Therapy: Where Do We Stand in 2023?
Lundstrom K. Lundstrom K. Viruses. 2023 Mar 7;15(3):698. doi: 10.3390/v15030698. Viruses. 2023. PMID: 36992407 Free PMC article. Review.
References
- Hodge JW, Grosenbach DW, Aarts WM, Poole DJ, Schlom J. Vaccine therapy of established tumors in the absence of autoimmunity. Clin Cancer Res. 2003;9:1837–49. - PubMed
- Essajee S, Kaufman HL. Poxvirus vaccines for cancer and HIV therapy. Expert Opin Biol Ther. 2004;4:575–88. - PubMed
- Karsten U, von Mensdorff-Pouilly S, Goletz S. What makes MUC1 a tumor antigen? Tumour Biol. 2005;26:217–20. - PubMed
- Duraisamy S, Kufe T, Ramasamy S, Kufe D. Evolution of the human MUC1 oncoprotein. Int J Oncol. 2007;31:671–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous