Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia - PubMed (original) (raw)
Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia
Yuliya Pylayeva-Gupta et al. Cancer Cell. 2012.
Abstract
Stromal responses elicited by early stage neoplastic lesions can promote tumor growth. However, the molecular mechanisms that underlie the early recruitment of stromal cells to sites of neoplasia remain poorly understood. Here, we demonstrate an oncogenic Kras(G12D)-dependent upregulation of GM-CSF in mouse pancreatic ductal epithelial cells (PDECs). An enhanced GM-CSF production is also observed in human PanIN lesions. Kras(G12D)-dependent production of GM-CSF in vivo is required for the recruitment of Gr1(+)CD11b(+) myeloid cells. The suppression of GM-CSF production inhibits the in vivo growth of Kras(G12D)-PDECs, and, consistent with the role of GM-CSF in Gr1(+)CD11b(+) mobilization, this effect is mediated by CD8(+) T cells. These results identify a pathway that links oncogenic activation to the evasion of antitumor immunity.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Figures
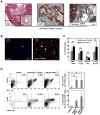
Figure 1. Orthotopic implantation of _KrasG12D_-PDEC generates a robust immune response
(A) Sections from orthotopic pancreatic grafts formed by _GFP-KrasG12D_-PDEC at 4 weeks post-implantation were stained with hematoxylin and eosin (H&E, scale bar, 500μm; inset scale bar, 25μm); anti-GFP antibody (scale bar, 50μm) and Trichrome blue (scale bar, 100μm). White arrowheads indicate GFP-positive neoplastic pancreatic ducts; black asterisk indicates neoplastic ducts; Ac stands for acinar compartment. (B) Immunofluorescence staining for CD45 and CK19 in pancreata of normal sham-injected control and orthotopic _GFP-KrasG12D_-PDEC animals (4 weeks post implantation). CK19 was used to identify ductal epithelia, CD45 was used to identify immune cells and nuclei were counterstained with DAPI. White asterisk indicates pancreatic ductal structures (left panel) and grafted ductal structures (right panel); white arrowheads indicate CD45+ cells. Scale bar, 100μm. (C) Percentage of immune cell types in pancreata was determined by flow cytometry of pancreatic tissue and quantified in the graph on the right. After gating on the CD45+ population, cells were analyzed for the presence of respective lineage markers (percent of each immune cell subtype out of total number of live cells sorted from the pancreas is shown). Error bars indicate SD, (n = 3–8 mice per group). (D) Flow cytometry analysis of pancreatic tissue for the presence of Gr1+CD11b+ myeloid and Foxp3+CD25+ Tregs. (Top) After gating on the CD45+ population, cells were analyzed for the presence of Gr1+CD11b+ subpopulation. The graph shows the percent Gr1+CD11b+ cells out of total number of live cells sorted from the pancreas. (Bottom) After gating on CD45+CD3+ T cells, cells were gated on CD4+ to examine intracellular Foxp3 versus surface CD25 staining. The graph shows the percent Tregs out of total number of CD4+ T cells. Representative flow cytometry plots are shown. Error bars indicate SD; INS–insufficient number of cells for analysis, (n = 4–8 mice per group). p value: *<0.05; **<0.01; NS–not significant. See also Figure S1.
Figure 2. GM-CSF is upregulated in _KrasG12D_-PDEC as well as PanIN-harboring mouse and human pancreata
(A) Levels of GM-CSF mRNA (black bar) and protein (gray bar) in _GFP-KrasG12D_- PDEC were assessed by quantitative RT-PCR and ELISA respectively. Data is presented as an average fold induction over values from isogenic _GFP-_WT-PDEC. Error bars indicate SD, (n=3). (B) Normalized expression of GM-CSF mRNA _GFP-KrasG12D_-PDEC (black bars) after24 hour treatment with DMSO, MAPK inhibitor U0126 (2μM) or PI3K inhibitor LY294002(10μM) was analyzed by quantitative RT-PCR. Error bars indicate SD, (n=3). (C) Levels of GM-CSF protein in pancreata grafted with _GFP-KrasG12D_-PDEC or pancreata from p48;LSL-KrasG12D mice. Data is presented as an average fold induction over values from normal pancreatic tissue. Error bars indicate SD, (n=3). (D) Immunohistochemical staining for GM-CSF protein in representative samples of human pancreatic cancer containing PanIN lesions. (a–normal duct from adjacent non-malignant tissue; b and c - PanIN lesions; d–invasive PDA). White arrowheads indicate pancreatic duct (a), PanIN (b and c) and PDA (d). Scale bar, 50μm.See also Figure S2.
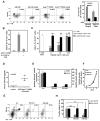
Figure 3. _KrasG12D_-PDEC promote accumulation of immunosuppressive Gr1+CD11b+ cells in a GM-CSF dependent manner
(A) Flow cytometry analysis of LinnegCD34+ hematopoietic progenitor cells for the surface markers CD11b and Gr1 following co-culture with GM-CSF or _GFP-KrasG12D_-PDEC with or without α-GM-CSF antibody (α-GM). Representative flow cytometry plots and a graph indicating percent of the Gr1+CD11b+ cells out of total number of live cells are shown. Error bars indicate SD, (n=3). (B) Quantification of BrdU+CD3+ T cells treated as indicated. T cells and Gr1+CD11b+ cells were co-cultured at a 1:1 ratio. Error bars indicate SD, (n=3). (C) Representative quantification of BrdU+CD3+ T cells co-cultured with either Gr1− CD11b+ or Gr1+CD11b+ cells isolated from mouse pancreata 8 weeks after injection with _GFP-KrasG12D_-PDEC. For proliferation assays, myeloid cells and T cells were cultured at ratios of 1:1, 1:5 or 1:10 respectively. T cells incubated in αCD3-coated wells and in the presence of αCD28 serve as control. Error bars indicate SD, (n=3). (D) Expression of mouse GM-CSF in the sera of either uninjected mice (control, n=4), or mice with _GFP-KrasG12D_-PDEC grafts 8 weeks post-implantation (n=5) was measured using ELISA. Each symbol represents a mouse, mean values for each group are represented by black lines. (E) Relative expression of GM-CSF mRNA (gray bars, left axis) and protein (black bars, right axis) in _GFP-KrasG12D_-PDEC 4 days after infection with lentiviruses containing either scrambled shRNA (scr) or GM-CSF shRNAs (GM-sh1, GM-sh2) was assessed by quantitative RT-PCR and ELISA. Error bars indicate SD, (n=3). (F) Growth analysis of scr (circles), GM-sh1 (squares) and GM-sh2 _GFP-KrasG12D_- PDEC (triangles) was assessed by MTT assay. Error bars indicate SD, (n=3). (G) Representative flow cytometry plots of pancreatic immune cells for the surface expression of Gr1 and CD11b markers at 4-weeks post-implantation of scr-, GM-sh1- and GM-sh2 _GFP-KrasG12D_-PDEC. After gating on the CD45+ population, cells were analyzed for the presence of CD11b+ and Gr1+ populations. The graph shows the percent Gr1+CD11b+ cells out of total number of live cells sorted from the pancreas. (H) Quantification of relative abundance of Gr1+CD11b+ and Gr1−CD11b+ cell populations is presented as a percent change in CD45+ single-positive Gr1−CD11b+ cells (black bars) and double-positive Gr1+CD11b+ population (gray bars) relative to scr _GFP-KrasG12D_-PDEC. Error bars indicate SD, (n= 6 mice per group). p value: **<0.01; ***<0.001, NS - not significant.
Figure 4. Functional consequence of ablating GM-CSF on growth of _KrasG12D_- PDEC in vivo
(A) Gross anatomical view of scr- and GM-sh _GFP-KrasG12D_-PDEC grafts (top, dotted outlines and arrows) at 8 weeks post-implantation. Scale bar, 5mm. Quantification of the graft size at 8 weeks post-implantation (gray bars, left axis) and percentage of overall lesion frequency (black bars, right axis) is indicated in the graph. Error bars indicate SD, (n=3). Sections from pancreata containing scr- and GM-sh _GFP-KrasG12D_-PDEC orthotopic grafts at 4 weeks post-implantation were stained with H&E (bottom, lesions are delineated by dotted outlines). Scale bar, 500μm. (B) Immunohistochemical staining for GFP on sections of scr- and GM-sh _GFP-KrasG12D_-PDEC orthotopic grafts at 1 and 2 weeks post-implantation. Red asterisks indicate engrafted area. Scale bar, 100μm. p value: *<0.05. See also Figure S3.
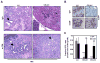
Figure 5. Engraftment of GM-CSF knock-down _KrasG12D_-PDEC is accompanied by an increase in infiltrating CD8+ T cells
(A) Immunohistochemical staining and quantification of CD8 and cleaved caspase 3 staining on sections of scr- and GM-sh _GFP-KrasG12D_-PDEC orthotopic grafts at 2 weeks post-implantation. CD8+ or cleaved caspase3-positive cells within the boundaries of orthotopic grafts were counted per field of view (FOV) at 20x magnification. White arrowheads indicate CD8+ cells; black arrowheads indicate caspase-3-positive cells. Scale bars, 50μm. Error bars indicate SD, (n = 4 mice per group, 4 FOV per mouse). (B) Immunohistochemical staining for CD8 and B220 on consecutive sections of scr- and GM-sh _GFP-KrasG12D_-PDEC orthotopic grafts at 4 weeks post-implantation. Numbers of CD8+ cells were counted per field of view (FOV) at 20x magnification and are shown in the graph. Arrows indicate co-aggregation of CD8+ cells and B220+ cells. Scale bar, 100μm. Error bars indicate SD, (n = 3 mice per group, 5 FOV per mouse). p value: ***<0.001. See also Figure S4.
Figure 6. CD8+ T cells are instrumental in the clearance of GM-CSF knock-down _KrasG12D_-PDEC
(A) Orthotopic grafts formed by scr- or GM-sh _GFP-KrasG12D_-PDEC implanted into either mock-depleted (IgG) or CD8-depleted animals were analyzed at 2 weeks post-implantation by H&E. Black arrowheads indicate pancreatic ductal structures. Scale bar, 100μm. (B) The extent of colonization of the grafted areas by scr- or GM-sh _GFP-KrasG12D_-PDEC in mock-depleted (IgG) or CD8-depleted animals at 2 weeks post-implantation was analyzed by immunohistochemistry for GFP. Scale bar, 100μm. (C) Graph depicts quantification of the data from (B) and indicates the fraction of GFP+ area per total area of the graft. Error bars indicate SD, (n = 4 mice per group, 5 FOV per mouse) p value: ***<0.001, NS–not significant. See also Figure S5.
Comment in
- Pancreatic cancer: The role of GM-CSF in pancreatic cancer unveiled.
Greenhill C. Greenhill C. Nat Rev Gastroenterol Hepatol. 2012 Aug;9(8):426. doi: 10.1038/nrgastro.2012.127. Epub 2012 Jun 26. Nat Rev Gastroenterol Hepatol. 2012. PMID: 22733349 No abstract available. - Suppressing the rejection of pancreatic tumours.
Alderton GK. Alderton GK. Nat Rev Cancer. 2012 Jul 19;12(8):510-1. doi: 10.1038/nrc3329. Nat Rev Cancer. 2012. PMID: 22810812 No abstract available. - Tumour immunology: Suppressing the rejection of pancreatic tumours.
Alderton GK. Alderton GK. Nat Rev Immunol. 2012 Jul 20;12(8):555. doi: 10.1038/nri3267. Nat Rev Immunol. 2012. PMID: 22814510 No abstract available.
Similar articles
- Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer.
Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, Vonderheide RH. Bayne LJ, et al. Cancer Cell. 2012 Jun 12;21(6):822-35. doi: 10.1016/j.ccr.2012.04.025. Cancer Cell. 2012. PMID: 22698406 Free PMC article. - Kras(G12D) induces EGFR-MYC cross signaling in murine primary pancreatic ductal epithelial cells.
Diersch S, Wirth M, Schneeweis C, Jörs S, Geisler F, Siveke JT, Rad R, Schmid RM, Saur D, Rustgi AK, Reichert M, Schneider G. Diersch S, et al. Oncogene. 2016 Jul 21;35(29):3880-6. doi: 10.1038/onc.2015.437. Epub 2015 Nov 23. Oncogene. 2016. PMID: 26592448 Free PMC article. - A Listeria vaccine and depletion of T-regulatory cells activate immunity against early stage pancreatic intraepithelial neoplasms and prolong survival of mice.
Keenan BP, Saenger Y, Kafrouni MI, Leubner A, Lauer P, Maitra A, Rucki AA, Gunderson AJ, Coussens LM, Brockstedt DG, Dubensky TW Jr, Hassan R, Armstrong TD, Jaffee EM. Keenan BP, et al. Gastroenterology. 2014 Jun;146(7):1784-94.e6. doi: 10.1053/j.gastro.2014.02.055. Epub 2014 Mar 6. Gastroenterology. 2014. PMID: 24607504 Free PMC article. - Identification and manipulation of biliary metaplasia in pancreatic tumors.
Delgiorno KE, Hall JC, Takeuchi KK, Pan FC, Halbrook CJ, Washington MK, Olive KP, Spence JR, Sipos B, Wright CV, Wells JM, Crawford HC. Delgiorno KE, et al. Gastroenterology. 2014 Jan;146(1):233-44.e5. doi: 10.1053/j.gastro.2013.08.053. Epub 2013 Aug 30. Gastroenterology. 2014. PMID: 23999170 Free PMC article. - Critical role of oncogenic KRAS in pancreatic cancer (Review).
Liu J, Ji S, Liang C, Qin Y, Jin K, Liang D, Xu W, Shi S, Zhang B, Liu L, Liu C, Xu J, Ni Q, Yu X. Liu J, et al. Mol Med Rep. 2016 Jun;13(6):4943-9. doi: 10.3892/mmr.2016.5196. Epub 2016 Apr 27. Mol Med Rep. 2016. PMID: 27121414 Review.
Cited by
- Immune Therapy in GI Malignancies: A Review.
Wang J, Reiss KA, Khatri R, Jaffee E, Laheru D. Wang J, et al. J Clin Oncol. 2015 Jun 1;33(16):1745-53. doi: 10.1200/JCO.2015.60.7879. Epub 2015 Apr 27. J Clin Oncol. 2015. PMID: 25918295 Free PMC article. Review. - A case of resected anaplastic carcinoma of the pancreas producing granulocyte-colony stimulating factor with literature review.
Kubo N, Suzuki S, Seki T, Furuke S, Yagi N, Ooki T, Aihara R, Mogi A, Yoshida Y, Kashiwabara K, Hosouchi Y, Shirabe K. Kubo N, et al. Surg Case Rep. 2024 Sep 5;10(1):205. doi: 10.1186/s40792-024-02008-3. Surg Case Rep. 2024. PMID: 39231851 Free PMC article. - Radiation promotes invasiveness of non-small-cell lung cancer cells through granulocyte-colony-stimulating factor.
Cui YH, Suh Y, Lee HJ, Yoo KC, Uddin N, Jeong YJ, Lee JS, Hwang SG, Nam SY, Kim MJ, Lee SJ. Cui YH, et al. Oncogene. 2015 Oct 16;34(42):5372-82. doi: 10.1038/onc.2014.466. Epub 2015 Feb 2. Oncogene. 2015. PMID: 25639867 - Yes-associated protein mediates immune reprogramming in pancreatic ductal adenocarcinoma.
Murakami S, Shahbazian D, Surana R, Zhang W, Chen H, Graham GT, White SM, Weiner LM, Yi C. Murakami S, et al. Oncogene. 2017 Mar 2;36(9):1232-1244. doi: 10.1038/onc.2016.288. Epub 2016 Aug 22. Oncogene. 2017. PMID: 27546622 Free PMC article. - Control of CD8 T-Cell Infiltration into Tumors by Vasculature and Microenvironment.
Peske JD, Woods AB, Engelhard VH. Peske JD, et al. Adv Cancer Res. 2015;128:263-307. doi: 10.1016/bs.acr.2015.05.001. Epub 2015 Jun 1. Adv Cancer Res. 2015. PMID: 26216636 Free PMC article. Review.
References
- Agbunag C, Lee KE, Buontempo S, Bar-Sagi D. Pancreatic duct epithelial cell isolation and cultivation in two-dimensional and three-dimensional culture systems. Methods in enzymology. 2006;407:703–710. - PubMed
- Barreda DR, Hanington PC, Belosevic M. Regulation of myeloid development and function by colony stimulating factors. Dev Comp Immunol. 2004;28:509–554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA016087/CA/NCI NIH HHS/United States
- R56 CA055360/CA/NCI NIH HHS/United States
- CA055360/CA/NCI NIH HHS/United States
- R01 CA055360/CA/NCI NIH HHS/United States
- 5 P30CA016087-31/CA/NCI NIH HHS/United States
- R37 CA055360/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous