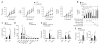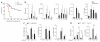The p110δ isoform of the kinase PI(3)K controls the subcellular compartmentalization of TLR4 signaling and protects from endotoxic shock - PubMed (original) (raw)
. 2012 Nov;13(11):1045-1054.
doi: 10.1038/ni.2426. Epub 2012 Sep 30.
Salma Taboubi 1, David Torres # 2, Sandrine Delbauve # 2, Abderrahman Hachani 3, Maria A Whitehead 1, Wayne P Pearce 1, Inma M Berenjeno 1, Gemma Nock 1, Alain Filloux 3, Rudi Beyaert 4 5, Veronique Flamand 2, Bart Vanhaesebroeck 1
Affiliations
- PMID: 23023391
- PMCID: PMC4018573
- DOI: 10.1038/ni.2426
The p110δ isoform of the kinase PI(3)K controls the subcellular compartmentalization of TLR4 signaling and protects from endotoxic shock
Ezra Aksoy et al. Nat Immunol. 2012 Nov.
Erratum in
- Nat Immunol. 2013 Aug;14(8):877. Berenjeno-Martin, Inma [corrected to Berenjeno, Inma M]
Abstract
Lipopolysaccharide activates plasma-membrane signaling and endosomal signaling by Toll-like receptor 4 (TLR4) through the TIRAP-MyD88 and TRAM-TRIF adaptor complexes, respectively, but it is unclear how the signaling switch between these cell compartments is coordinated. In dendritic cells, we found that the p110δ isoform of phosphatidylinositol-3-OH kinase (PI(3)K) induced internalization of TLR4 and dissociation of TIRAP from the plasma membrane, followed by calpain-mediated degradation of TIRAP. Accordingly, inactivation of p110δ prolonged TIRAP-mediated signaling from the plasma membrane, which augmented proinflammatory cytokine production while decreasing TRAM-dependent endosomal signaling that generated anti-inflammatory cytokines (interleukin 10 and interferon-β). In line with that altered signaling output, p110δ-deficient mice showed enhanced endotoxin-induced death. Thus, by controlling the 'topology' of TLR4 signaling complexes, p110δ balances overall homeostasis in the TLR4 pathway.
Figures
Figure 1
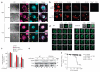
The p110δ isoform of PI(3)K is recruited to the LPS-activated TLR4 complex and controls TLR4 internalization in DCs. (a) Surface expression of TLR4 on wild-type (WT) and δ(D910A) BMDCs left unstimulated (Med) or stimulated with LPS (100 ng/ml), analyzed by flow cytometry either at 120 min (left) or over a time course of 0–120 min (right). Ctrl Ab, isotype-matched control antibody. (b) Surface expression of TLR4 on wild-type and δ(D910A) splenic DCs (splenocytes after enrichment for CD11c+ cells) left unstimulated (Med) or stimulated for 2 h with LPS (1 μg/ml), analyzed by flow cytometry. (c) PI(3)K lipid-kinase assay of proteins immunoprecipitated from untreated (Med; top) or LPS-treated (bottom) wild-type and δ(D910A) BMDCs with antibodies to various PI(3)K isoforms (horizontal axes). (d) Immunoblot analysis of Akt phosphorylated at Thr308 (p-Akt (T308)) or Ser473 (p-Akt (S473)) and total Akt in wild-type BMDCs treated with vehicle (Veh) or IC87114, and δ(D910A) BMDCs treated with vehicle, and stimulated for 0–60 min (below lanes) with LPS (100 ng/ml). Right margin, molecular size (in kDa). (e) Immunoblot analysis of phosphorylated and total Akt in BMDCs (genotype, above lanes) stimulated with LPS as in d. (f) Immunoprecipitation (IP) and immunoblot analysis (IB) of untreated (−) or LPS-treated (+) wild-type, δ(D910A) and _Tlr4_−/− BMDCs, assessed with anti-p110δ, anti-TLR4, anti-MyD88 or anti-TRAM. (g) Immunoassay (as in f) of wild-type and δ(D910A) BMDCs treated for 0–10 min (below lanes) with LPS, assessed with anti-p110δ, anti-TLR4, anti-MyD88 or anti-TIRAP. *P < 0.01, **P < 0.001 and ***P < 0.0001 (Student’s _t_-test). Data represent one of three experiments (a; mean ± s.d. (right) of three to four mice per group), four experiments (b; mean and s.d. of three to four mice per group), three experiments (c; mean and s.d. of three mice per group), one of three experiments (d,e) or at least two experiments with two to three mice per group (f,g).
Figure 2
The activity of p110δ in BMDCs is involved in the dissociation of TIRAP from the plasma membrane after stimulation with LPS. (a) Confocal microscopy of wild-type and δ(D910A) BMDCs left untreated (Med) or activated for 20 min with LPS and then stained with anti-TIRAP or GST–PLC-δ-PH, followed by anti-GST (to probe for PtdIns(4,5)P2); arrowheads indicate the localization of TIRAP with PtdIns(4,5)P2 at the plasma membrane. DIC, differential interference contrast. Scale bars, 10 μm. (b) Confocal microscopy of wild-type BMDCs (left group) pretreated for 1 h with vehicle (DMSO) or IC87114 (1 μM) and then stimulated for 0–60 min (above images) with LPS (100 ng/ml), and of a _Tirap_−/− BMDC treated with vehicle and left unstimulated (far right). Arrowheads indicate localization of TIRAP at the cell periphery. Original magnification, ×63. (c) Frequency of BMDCs (n = 50–60, collected from ten fields) with TIRAP staining at the plasma membrane, assessed by differential interference contrast microscopy (treated, stained and analyzed as in a,b). *P < 0.01 and **P < 0.001 (Student’s _t_-test). (d) Video microscopy of wild-type and δ(D910A) BMDCs transfected to express GFP-TIRAP and then imaged for 15 min in medium alone before stimulation with LPS (100 ng/ml), followed by imaging for 18 h (time, top right corners; ‘0 min’ indicates 1 min after the addition of LPS). Original magnification, ×63. (e) Immunoblot analysis of lysates of wild-type and δ(D910A) BMDCs stimulated for 0 or 15 min with LPS (100 ng/ml), followed by subcellular fractionation of the membrane (M) and cytosol (C). (f) PI(3)K kinase assay of the binding of PtdIns(4,5)P2 to TIRAP in plates coated with PtdIns(4,5)P2 and preincubated with GST-TIRAP, followed by the addition of TIRAP alone ((1) TIRAP), TIRAP plus recombinant p110δ-p85 fusion protein ((2) TIRAP + p110δ), TIRAP plus p110δ-p85 without ATP ((2) − ATP) and TIRAP plus p110δ-p85 preincubated with IC87114 (1 μM) ((2) + IC87114)); results are presented as absorbance at 450 nm (_A_450). Data are from one experiment with five mice per group (a; one image of at least ten per condition), one experiment representative of three (b,e,f; with triplicates in f) or three experiments with two to three mice per strain (c; mean and s.d.) or two mice per strain (d; one image of at least two to three per condition).
Figure 3
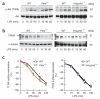
PTEN regulates LPS-induced TIRAP degradation and TLR4 internalization in BMDCs, but SHIP does not. (a) Immunoblot analysis of phosphorylated and total Akt in wild-type, Pten+/− and SHIP-1-deficient (_Inpp5d_−/−) BMDCs treated for 0–60 min (below lanes) with LPS (100 ng/ml). (b) Immunoblot analysis of TIRAP and α-tubulin (loading control) in lysates of wild-type, Pten+/− and SHIP-1-deficient BMDCs treated for 0–60 min (below lanes) with LPS. (c) Surface expression of TLR4 on wild-type, Pten+/− and SHIP-1-deficient BMDCs stimulated for 0–120 min (horizontal axis) with LPS (100 ng/ml), analyzed by flow cytometry. *P < 0.01 (Student’s _t_-test). Data are from one experiment representative of three (a), one experiment representative of two with three to four mice per group (b) or three experiments (c; mean ± s.d. of three mice per group).
Figure 4
‘Licensing’ of calpain-induced TIRAP proteolysis by p110δ in BMDCs and J774 macrophages. (a) Immunoblot analysis of TIRAP and α-tubulin in lysates of wild-type and δ(D910A) BMDCs pretreated for 1 h with vehicle or N-ALLN (50 μM) and then activated for 0–60 min (below lanes) with LPS (100 ng/ml). (b) Immunoblot analysis of TIRAP and α-tubulin in lysates of wild-type BMDCs and BMDCs with transgenic expression of calpastatin (calp-TG), treated for 0–60 min (below lanes) with LPS. (c) Immunoprecipitation and immunoblot analysis of J774 macrophages left untransfected (−) or transfected for 36 h to express Flag-tagged TIRAP (+), then pretreated for 1 h with vehicle (DMSO) or IC87114 (1 μM) and then left unstimulated or stimulated for 10 min with LPS (1 μg/ml), assessed with anti-Flag or anti-calpain-1 (above), along with immunoblot analysis of total cell lysates (TCL; below). (d) Immunoblot analysis of recombinant GST-TIRAP incubated with calpain-1 or calpain-2 (below lanes) in various assay conditions (above lanes). §, full-length TIRAP; §§, §§§ and §§§§, TIRAP cleavage products. (e) Immunoblot analysis of wild-type and δ(D910A) BMDCs left unstimulated (0 min) or stimulated for 10 min with LPS (100 ng/ml), followed by subcellular fractionation of membrane and cytosol. FL, full-length. Data are from one experiment representative of three with two to three mice per group (a,b) or one experiment representative of three (c–e).
Figure 5
Inhibition of p110δ in BMDCs augments the production of proinflammatory cytokines while inhibiting IFN-β and the products of interferon-stimulated genes. (a) Cytokine concentrations in supernatants of wild-type and δ(D910A) BMDCs stimulated for 24 h with various concentrations of LPS (horizontal axes). *P < 0.05 and **P < 0.005 (Mann-Whitney _U_-test). (b) IL-12p40 concentrations in culture supernatant of wild-type and δ(D910A) BMDCs pretreated for 1 h with various PI(3)K inhibitors (below), followed by stimulation for 24 h with LPS (100 ng/ml). *P < 0.05 (Mann-Whitney _U_-test). (c) IL-12p40 concentration in supernatants of wild-type and δ(D910A) splenocytes left unstimulated (Med) or stimulated with LPS (100 ng/ml). *P < 0.05 (Mann-Whitney _U_-test). (d) IL-12p40 concentration in wild-type and δ(D910A) BMDCs left unstimulated (None) or stimulated with various TLR ligands (horizontal axis) or IL-1β (20 ng/ml) or TNF (20 ng/ml). SM, S. minnesota; EC, E. coli; MPLA, monophosphoryl lipid A; LTA, lipoteichoic acid. *P < 0.05 (Mann-Whitney _U_-test). (e) Concentration of IFN-β and RANTES in supernatant of wild-type and δ(D910A) BMDCs, as well as wild-type BMDCs treated with IC87114 (0.5 μM; WT + IC87114), left unstimulated (Med) or stimulated with LPS or monophosphoryl lipid A. U, undetectable. *P < 0.05 (analysis of variance). (f) Concentration of IFN-β and IL-10 in supernatants of wild-type and δ(D910A) splenocytes left unstimulated (Med) or stimulated with LPS (100 ng/ml). *P < 0.05 (Mann-Whitney _U_-test). Data are representative of three experiments (a–d; mean ± s.d. of five mice per group in a; mean and s.d. of five to six mice per group in b; mean and s.d. in c; and mean and s.d. of five mice per group in d) or two experiments (e,f; mean and s.d. of five mice per group (e) or five to six mice per group (f)).
Figure 6
The kinase activity of p110δ in BMDCs is required for optimal IFN-β production through IRF3 and late activation of NF-κB and p38. (a) Immunoblot analysis (top) of phosphorylated and total p38 and IKKα-IKKβ in extracts of wild-type BMDCs treated with vehicle (Veh) or IC87114, and δ(D910A) BMDCs treated with vehicle, stimulated for 0–60 min (below lanes) with LPS (100 ng/ml); below, ratio of phosphorylated protein to total protein. Vertical dotted line (top) indicates cropped lanes compiled from immunoblots of lysates analyzed simultaneously under the same conditions. (b) Confocal microscopy (left) of wild-type and δ(D910A) BMDCs left unstimulated or stimulated for 45 min with LPS (100 ng/ml), stained with the DNA-intercalating dye DAPI (staining nuclei) and for NF-κB p65 (p65-RelA); and frequency of BMDCs with only p65+ nuclei (n = 50 cells for each of three mice per group; right). Scale bars (left), 10 μm. *P < 0.01 (Student’s _t_-test). (c) Immunoblot analysis of phosphorylated and total p38 and IRF3 in wild-type BMDCs treated with vehicle (DMSO), IC87114, U73221 or Dynasore, then stimulated for 0–180 min with LPS (10 ng/ml). Vertical dotted lines, as in a. (d) Immunoblot analysis of phosphorylated and total IRF3 in wild-type and δ(D910A) BMDCs stimulated for 0–180 min with LPS (10 ng/ml). (e) Confocal microscopy (left) of IRF3 and DAPI staining in BMDCs as in b; and frequency of BMDCs with only IRF3+ nuclei (n = 50 cells for each of three mice per group; right). Scale bars, 10 μm. **P < 0.01 by Student’s _t_-test (right). Data are from one representative of three experiments with three to four mice (a, top) or two to three experiments with two to three mice (c,d) or are representative of one experiment (a, bottom; mean and s.e.m. of three mice per group) or three experiments (b,e; mean and s.d.).
Inhibition of p110δ in mice augments endotoxinmediated death. (a) Kaplan-Meier analysis of the survival of wild-type and δ(D910A) mice (n = 15 per group) injected intraperitoneally with LPS (from E. coli strain O55:B5; 30 mg per kg body weight). P < 0.0001 (log-rank test). (b) Cytokine concentrations in serum from mice treated as in a. *P < 0.05 and **P < 0.005 (Mann-Whitney _U_-test). (c) Cytokine mRNA in spleens of mice treated as in a, presented relative to the abundance of HPRT mRNA (encoding hypoxanthine guanine phosphoribosyl transferase). *P < 0.05 and **P < 0.005 (Mann-Whitney _U_-test). (d) Plasma concentration of IFN-β and IL-10 in serum from wild-type, IRF3- and IL-10-deficient mice injected intraperitoneally with LPS (from E. coli strain 0111:B4; 10 mg per kg body weight). *P < 0.05, **P < 0.005 and ***P < 0.005 (Mann-Whitney _U_-test). Data are representative of two experiments (mean and s.d. of three to five mice per group in b–d).
Comment in
- Signalling: PI3Kδ keeps TLR4 signalling on track.
Papatriantafyllou M. Papatriantafyllou M. Nat Rev Immunol. 2012 Nov;12(11):744. doi: 10.1038/nri3330. Epub 2012 Oct 12. Nat Rev Immunol. 2012. PMID: 23059427 No abstract available. - Balancing pro- and anti-inflammatory TLR4 signaling.
Siegemund S, Sauer K. Siegemund S, et al. Nat Immunol. 2012 Nov;13(11):1031-3. doi: 10.1038/ni.2452. Nat Immunol. 2012. PMID: 23080196 No abstract available.
Similar articles
- B and T lymphocyte attenuator inhibits LPS-induced endotoxic shock by suppressing Toll-like receptor 4 signaling in innate immune cells.
Kobayashi Y, Iwata A, Suzuki K, Suto A, Kawashima S, Saito Y, Owada T, Kobayashi M, Watanabe N, Nakajima H. Kobayashi Y, et al. Proc Natl Acad Sci U S A. 2013 Mar 26;110(13):5121-6. doi: 10.1073/pnas.1222093110. Epub 2013 Mar 11. Proc Natl Acad Sci U S A. 2013. PMID: 23479601 Free PMC article. - Annexin A2 binds to endosomes and negatively regulates TLR4-triggered inflammatory responses via the TRAM-TRIF pathway.
Zhang S, Yu M, Guo Q, Li R, Li G, Tan S, Li X, Wei Y, Wu M. Zhang S, et al. Sci Rep. 2015 Nov 3;5:15859. doi: 10.1038/srep15859. Sci Rep. 2015. PMID: 26527544 Free PMC article. - Roles for LPS-dependent interaction and relocation of TLR4 and TRAM in TRIF-signaling.
Tanimura N, Saitoh S, Matsumoto F, Akashi-Takamura S, Miyake K. Tanimura N, et al. Biochem Biophys Res Commun. 2008 Mar 28;368(1):94-9. doi: 10.1016/j.bbrc.2008.01.061. Epub 2008 Jan 28. Biochem Biophys Res Commun. 2008. PMID: 18222170 - TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling.
Ciesielska A, Matyjek M, Kwiatkowska K. Ciesielska A, et al. Cell Mol Life Sci. 2021 Feb;78(4):1233-1261. doi: 10.1007/s00018-020-03656-y. Epub 2020 Oct 15. Cell Mol Life Sci. 2021. PMID: 33057840 Free PMC article. Review. - [TIR domain--containing adaptors regulate TLR-mediated signaling pathways].
Yamamoto M, Akira S. Yamamoto M, et al. Nihon Rinsho. 2004 Dec;62(12):2197-203. Nihon Rinsho. 2004. PMID: 15597785 Review. Japanese.
Cited by
- Microbe-inducible trafficking pathways that control Toll-like receptor signaling.
Tan Y, Kagan JC. Tan Y, et al. Traffic. 2017 Jan;18(1):6-17. doi: 10.1111/tra.12454. Epub 2016 Nov 23. Traffic. 2017. PMID: 27731905 Free PMC article. Review. - Uncoupling of pro- and anti-inflammatory properties of Staphylococcus aureus.
Peres AG, Stegen C, Li J, Xu AQ, Levast B, Surette MG, Cousineau B, Desrosiers M, Madrenas J. Peres AG, et al. Infect Immun. 2015 Apr;83(4):1587-97. doi: 10.1128/IAI.02832-14. Epub 2015 Feb 2. Infect Immun. 2015. PMID: 25644014 Free PMC article. - An AP-3-dependent pathway directs phagosome fusion with Rab8 and Rab11 vesicles involved in TLR2 signaling.
Petnicki-Ocwieja T, Sharma B, Powale U, Pathak D, Tan S, Hu LT. Petnicki-Ocwieja T, et al. Traffic. 2022 Dec;23(12):558-567. doi: 10.1111/tra.12870. Epub 2022 Oct 24. Traffic. 2022. PMID: 36224049 Free PMC article. - Cathelicidins Modulate TLR-Activation and Inflammation.
Scheenstra MR, van Harten RM, Veldhuizen EJA, Haagsman HP, Coorens M. Scheenstra MR, et al. Front Immunol. 2020 Jun 9;11:1137. doi: 10.3389/fimmu.2020.01137. eCollection 2020. Front Immunol. 2020. PMID: 32582207 Free PMC article. Review. - A novel TLR2-triggered signalling crosstalk synergistically intensifies TNF-mediated IL-6 induction.
Chang YL, Chen TH, Wu YH, Chen GA, Weng TH, Tseng PH, Hsieh SL, Fu SL, Lin CH, Chen CJ, Chu CL, Chio II, Mak TW, Chen NJ. Chang YL, et al. J Cell Mol Med. 2014 Jul;18(7):1344-57. doi: 10.1111/jcmm.12294. Epub 2014 Apr 24. J Cell Mol Med. 2014. PMID: 24758719 Free PMC article.
References
- Janeway CA, Jr., Medzhitov R. Innate immune recognition. Annu. Rev. Immunol. 2002;20:197–216. - PubMed
- Akira S, Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. - PubMed
- Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125:943–955. - PubMed
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous