Identification of human epididymis protein-4 as a fibroblast-derived mediator of fibrosis - PubMed (original) (raw)
Identification of human epididymis protein-4 as a fibroblast-derived mediator of fibrosis
Valerie S LeBleu et al. Nat Med. 2013 Feb.
Abstract
The functional contribution of myofibroblasts in fibrosis is not well understood. Using a new genetic mouse model to track and isolate myofibroblasts, we performed gene expression profiling followed by biological validation to identify HE4 (encoding human epididymis protein 4, also known as WAP 4-disulfide core domain-2 or Wfdc2) as the most upregulated gene in fibrosis-associated myofibroblasts. The HE4 gene encodes for a putative serine protease inhibitor that is upregulated in human and mouse fibrotic kidneys and is elevated in the serum of patients with kidney fibrosis. HE4 suppresses the activity of multiple proteases, including serine proteases and matrix metalloproteinases, and specifically inhibits their capacity to degrade type I collagen. In particular, we identified two serine proteases, Prss35 and Prss23, as HE4 targets with functional relevance in kidney fibrosis. Administration of HE4-neutralizing antibodies accelerated collagen I degradation and inhibited fibrosis in three different mouse models of renal disease. Collectively these studies suggest that HE4 is a potential biomarker of renal fibrosis and a new therapeutic target.
Conflict of interest statement
Conflict of Interest: None
Figures
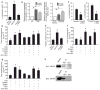
Figure 1. αSMA+ myofibroblasts accumulate in the interstitium and express HE4 in renal fibrosis
a. Visualization of αSMA-RFP+ cells in control (contralateral kidney to UUO) and fibrotic mouse kidneys (UUO) from α SMA-RFP transgenic mice. DAPI (blue): nuclei. Quantitation of αSMA+ cells per visual field in control and fibrotic kidney, evaluated at Day 10 post UUO. b. Visualization of αSMA-RFP+ myofibroblasts cultured from control kidneys (mouse normal fibroblasts, mNF) and fibrotic kidneys (mouse fibrosis-associated fibroblasts, mFAF). c. Relative HE4 gene expression in mFAF normalized to mNF. AU: Arbitrary Unit. d. Western blot for HE4 in mouse mNF and mFAF. Actin was used as an internal control. rHE4: recombinant HE4 protein used as positive control. e. Western blot for HE4 in mNF and mFAF culture media, with loading normalized to cell numbers. Bovine serum albumin (BSA) also controls for lane loading. f. Relative HE4 gene expression in control kidneys (n=5) and fibrotic kidneys (n=5), evaluated at Day 10 post UUO. g. Western blot for HE4 in mouse control and fibrotic kidneys. Actin was used as an internal control. Scale bar: 50μm. Data shown as mean +/− sem. *p<0.05.
Figure 2. HE4 is a pan-serine protease and MMP2/9 inhibitor which prevents type I collagen degradation
a. p-nitroalinine release in nM assessed by spectrophotometric readout of color development as a measure of serine protease activity in non-fibrotic control kidney lysates, fibrotic kidney lysates (UUO, Day 10), and fibrotic kidney lysates (UUO, Day 10) incubated with recombinant HE4 protein (rHE4). b. Relative Prss23 and Prss35 gene expression in mouse fibrosis-derived fibroblasts (mFAF) and mouse fibrotic kidneys (Fibrotic) compared and normalized to mouse normal fibroblasts (mNF, derived from non-fibrotic kidneys) and contralateral non-fibrotic kidneys (evaluated at Day 10 post UUO), respectively. nNFs and contralateral non-fibrotic kidneys were set arbitrarily to 1 (white bar). AU, arbitrary units. c. p-nitroalinine release in nM assessed by spectrophotometric readout of color development using Prss23 and Prss35 serine protease with and without recombinant HE4 (rHE4). d. Hydroxyproline release assay: free hydroxyproline (μg.ml−1) from type I collagen digestion: type I collagen digested by Prss23 or Prss35 with and without rHE4, with and without anti-HE4 antibody. e. Trypsin activity in non-fibrotic control kidney lysates, fibrotic (UUO, Day 10) kidneys, and fibrotic kidneys pre-treated with rHE4. f. Hydroxyproline release assay: free hydroxyproline (μg.ml−1) from type I collagen digestion: type I collagen digested by trypsin with and without HE4, with and without BSA, and with and without anti-HE4 antibody. g. Hydroxyproline release assay: free hydroxyproline (μg.ml−1) from type I collagen digestion: type I collagen digested by MMP2 or MMP9 with and without rHE4, with and without anti-HE4 antibody. h. Immunoprecipitation (IP) of fibrotic kidney lysates using IgG (control) or HE4 antibody and western blot for MMP2/9. Data shown as mean +/− sem. *p<0.05.
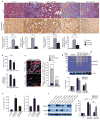
Figure 3. HE4 neutralization inhibits kidney fibrosis
a. Representative Masson Trichrome (MTS) and type I collagen staining of control and fibrotic kidneys from mice treated with anti-HE4 (UUO: n=5, NTN: n=6) or anti-IgG (UUO: n=5, NTN: n=5) antibody. Respective morphometric analyses for relative interstitial fibrosis based on MTS staining and type I collagen staining shows reduced fibrosis in anti-HE4-treated mice. AU: Arbitrary Unit. b. Blood urea nitrogen (BUN, mg.dL−1) and urine albumin/creatinine ratio measurements of mice with NTN and treated with anti-IgG (n=5) or anti-HE4 (n=6) antibodies. c. Immunolabeling for HE4 and αSMA in fibrotic kidneys from mice treated with anti-HE4 or anti-IgG control antibodies. DAPI (blue): nuclei. Histogram represents the relative number of αSMA+, HE4+, αSMA+/HE4+ (double positive) cells per field of view. d. Gelatin zymography using lysates of contralateral (normal) kidney, fibrotic kidneys of mice treated with anti-IgG, and fibrotic kidneys of mice treated with anti-HE4. Lysates are from kidneys at Day 10 post UUO. Histogram depicts relative band intensity normalized to actin western blot of samples loaded. e. Serine protease activity in kidney lysates (UUO) from fibrotic kidneys and fibrotic kidneys of mice treated with anti-HE4 (left). Trypsin activity in kidney lysates (UUO) from fibrotic kidneys and fibrotic kidneys of mice treated with anti-HE4 (right). Lysates are from kidneys at Day 10 post UUO. f. Western blot analyses for MMP2, MMP9 and actin loading control of lysates of contralateral (normal) kidney of mice treated with anti-IgG or anti-HE4 and of fibrotic kidneys of mice treated with anti-IgG or anti-HE4. Lysates are from kidneys at Day 10 post UUO. Histogram depicts relative band intensity normalized to actin. Scale bar: 50μm. Data shown as mean +/− sem. *p<0.05.
Figure 4. HE4 is elevated in human fibrotic kidneys, human fibrosis-associated fibroblasts and in serum of patients with renal fibrosis
a. Relative HE4 gene expression of different fibroblast cultures (normalized to normal fibroblast (NF, line TK173) set arbitrarily to 1). Fibrosis associated fibroblast (FAF) 1: TK274, FAF2: TK188, FAF3: TK239, FAF4: TK261, FAF5: TK257. AU, arbitrary units. b. Relative PRSS23 and PRSS35 gene expression of different fibroblast cultures (normalized to normal fibroblast (NF, line TK173) set arbitrarily to 1). AU, arbitrary units. c. Western blot analysis for HE4 in human NF (from left to right: line TK173, TK231a and TK163) and human FAF (from left to right line TK274, TK188, and TK239). Actin is used as internal control. d. Western blot for HE4 in human NF and FAF culture media, with loading normalized to cell numbers. Bovine serum albumin (BSA) also controls for lane loading. e. Immunolabeling for HE4 and αSMA in human kidneys with chronic kidney disease (Alport syndrome) and renal fibrosis. DAPI (blue): nuclei. f. Serum HE4 (sHE4) levels (pM) from healthy control (n=5) and CKD patients (n=11). Scale bar: 50μm. *p<0.05
Comment in
- Fibrosis: HE4--a biomarker and target in renal fibrosis.
Allison SJ. Allison SJ. Nat Rev Nephrol. 2013 Mar;9(3):124. doi: 10.1038/nrneph.2013.2. Epub 2013 Feb 12. Nat Rev Nephrol. 2013. PMID: 23399582 No abstract available. - Myofibroblasts, regeneration or renal fibrosis--is there a decisive hint?
Lindquist JA, Mertens PR. Lindquist JA, et al. Nephrol Dial Transplant. 2013 Nov;28(11):2678-81. doi: 10.1093/ndt/gft247. Epub 2013 Aug 23. Nephrol Dial Transplant. 2013. PMID: 23975753
Similar articles
- Silencing HE4 alleviates the renal fibrosis in lupus nephritis mice by regulating the C3/MMPs/prss axis.
Li Y, Zhong X, Yang F. Li Y, et al. Naunyn Schmiedebergs Arch Pharmacol. 2024 Jul;397(7):4823-4831. doi: 10.1007/s00210-023-02883-x. Epub 2023 Dec 29. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38157023 Free PMC article. - Myofibroblasts, regeneration or renal fibrosis--is there a decisive hint?
Lindquist JA, Mertens PR. Lindquist JA, et al. Nephrol Dial Transplant. 2013 Nov;28(11):2678-81. doi: 10.1093/ndt/gft247. Epub 2013 Aug 23. Nephrol Dial Transplant. 2013. PMID: 23975753 - HE4 Predicts Progressive Fibrosis and Cardiovascular Events in Patients With Dilated Cardiomyopathy.
Yamamoto M, Hanatani S, Araki S, Izumiya Y, Yamada T, Nakanishi N, Ishida T, Yamamura S, Kimura Y, Arima Y, Nakamura T, Takashio S, Yamamoto E, Sakamoto K, Kaikita K, Matsushita K, Morimoto S, Ito T, Tsujita K. Yamamoto M, et al. J Am Heart Assoc. 2021 Aug 3;10(15):e021069. doi: 10.1161/JAHA.120.021069. Epub 2021 Jul 29. J Am Heart Assoc. 2021. PMID: 34320813 Free PMC article. - Potential association between elevated serum human epididymis protein 4 and renal fibrosis: A systemic review and meta-analysis.
Chen P, Yang Q, Li X, Qin Y. Chen P, et al. Medicine (Baltimore). 2017 Sep;96(36):e7824. doi: 10.1097/MD.0000000000007824. Medicine (Baltimore). 2017. PMID: 28885334 Free PMC article. Review. - Molecular insights into renal interstitial fibrosis.
Eddy AA. Eddy AA. J Am Soc Nephrol. 1996 Dec;7(12):2495-508. doi: 10.1681/ASN.V7122495. J Am Soc Nephrol. 1996. PMID: 8989727 Review.
Cited by
- Redefining Immune Dynamics in Acute Pancreatitis: The Protective Role of Galectin-3 Deletion and Treg Cell Enhancement.
Milivojcevic Bevc I, Tasic-Uros D, Stojanovic BS, Jovanovic I, Dimitrijevic Stojanovic M, Gajovic N, Jurisevic M, Radosavljevic G, Pantic J, Stojanovic B. Milivojcevic Bevc I, et al. Biomolecules. 2024 May 30;14(6):642. doi: 10.3390/biom14060642. Biomolecules. 2024. PMID: 38927046 Free PMC article. - Silencing HE4 alleviates the renal fibrosis in lupus nephritis mice by regulating the C3/MMPs/prss axis.
Li Y, Zhong X, Yang F. Li Y, et al. Naunyn Schmiedebergs Arch Pharmacol. 2024 Jul;397(7):4823-4831. doi: 10.1007/s00210-023-02883-x. Epub 2023 Dec 29. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38157023 Free PMC article. - Human Epididymis Protein 4: A Novel Biomarker for Lupus Nephritis and Chronic Kidney Disease in Systemic Lupus Erythematosus.
Yang Z, Zhang Z, Qin B, Wu P, Zhong R, Zhou L, Liang Y. Yang Z, et al. J Clin Lab Anal. 2016 Nov;30(6):897-904. doi: 10.1002/jcla.21954. Epub 2016 Apr 13. J Clin Lab Anal. 2016. PMID: 27075413 Free PMC article. - Villus myofibroblasts are developmental and adult progenitors of mammalian gut lymphatic musculature.
Sanketi BD, Mantri M, Huang L, Tavallaei MA, Hu S, Wang MFZ, De Vlaminck I, Kurpios NA. Sanketi BD, et al. Dev Cell. 2024 May 6;59(9):1159-1174.e5. doi: 10.1016/j.devcel.2024.03.005. Epub 2024 Mar 26. Dev Cell. 2024. PMID: 38537630 - RNA-seq of serial kidney biopsies obtained during progression of chronic kidney disease from dogs with X-linked hereditary nephropathy.
Chu CP, Hokamp JA, Cianciolo RE, Dabney AR, Brinkmeyer-Langford C, Lees GE, Nabity MB. Chu CP, et al. Sci Rep. 2017 Dec 1;7(1):16776. doi: 10.1038/s41598-017-16603-y. Sci Rep. 2017. PMID: 29196624 Free PMC article.
References
- Zeisberg M, Strutz F, Muller GA. Role of fibroblast activation in inducing interstitial fibrosis. Journal of nephrology. 2000;13 (Suppl 3):S111–120. - PubMed
- Eddy AA. Molecular insights into renal interstitial fibrosis. J Am Soc Nephrol. 1996;7:2495–2508. - PubMed
- Strutz F, Muller GA. Renal fibrosis and the origin of the renal fibroblast. Nephrol Dial Transplant. 2006;21:3368–3370. - PubMed
- Okada H, Strutz F, Danoff TM, Kalluri R, Neilson EG. Possible mechanisms of renal fibrosis. Contributions to nephrology. 1996;118:147–154. - PubMed
- Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol. 21:1819–1834. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54 CA163191/CA/NCI NIH HHS/United States
- DK55001/DK/NIDDK NIH HHS/United States
- CA163191/CA/NCI NIH HHS/United States
- CA151925/CA/NCI NIH HHS/United States
- R01 DK081576/DK/NIDDK NIH HHS/United States
- CA155370/CA/NCI NIH HHS/United States
- 5T32HL007374-30/HL/NHLBI NIH HHS/United States
- T32 DK007760/DK/NIDDK NIH HHS/United States
- T32 GM007226/GM/NIGMS NIH HHS/United States
- DK081976/DK/NIDDK NIH HHS/United States
- U01 CA151925/CA/NCI NIH HHS/United States
- 2T32DK007760-11/DK/NIDDK NIH HHS/United States
- R01 CA125550/CA/NCI NIH HHS/United States
- CA125550/CA/NCI NIH HHS/United States
- R01 DK055001/DK/NIDDK NIH HHS/United States
- GM07226/GM/NIGMS NIH HHS/United States
- T32 HL007374/HL/NHLBI NIH HHS/United States
- R01 CA155370/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials