Signals from the lysosome: a control centre for cellular clearance and energy metabolism - PubMed (original) (raw)
Review
Signals from the lysosome: a control centre for cellular clearance and energy metabolism
Carmine Settembre et al. Nat Rev Mol Cell Biol. 2013 May.
Abstract
For a long time, lysosomes were considered merely to be cellular 'incinerators' involved in the degradation and recycling of cellular waste. However, now there is compelling evidence indicating that lysosomes have a much broader function and that they are involved in fundamental processes such as secretion, plasma membrane repair, signalling and energy metabolism. Furthermore, the essential role of lysosomes in autophagic pathways puts these organelles at the crossroads of several cellular processes, with significant implications for health and disease. The identification of a master regulator, transcription factor EB (TFEB), that regulates lysosomal biogenesis and autophagy has revealed how the lysosome adapts to environmental cues, such as starvation, and targeting TFEB may provide a novel therapeutic strategy for modulating lysosomal function in human disease.
Figures
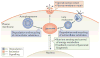
Figure 1. Main functions of the lysosome and their relationship with key cellular processes
Lysosomes are involved in the degradation and recycling of extracellular material, via endocytosis, and intracellular material, via autophagy. In these processes lysosomes fuse with late endosomes and with autophagosomes, respectively. The resulting breakdown products are used to generate new cellular components and energy in response to the nutritional needs of the cell. Lysosomes also undergo Ca2+ regulated exocytosis to secrete their content into the extracellular space and to repair damaged plasma membrane. Upon plasma membrane injury, lysosomes rapidly migrate to the damaged site and fuse with the plasma membrane to allow efficient resealing. More recently, lysosomes have been identified as signaling organelles that can sense nutrient availability and activate a lysosome-to-nucleus signaling pathway that mediates the starvation response and regulates energy metabolism.
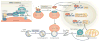
Figure 2. Model of TFEB regulation and function during starvation
This model illustrates how transcription factor EB (TFEB) is induced by starvation and mediates the starvation response by regulating lipid catabolism. In the presence of adequate nutrition TFEB interacts with the lysosome nutrient sensing (LYNUS) machinery, which senses lysosomal nutrient levels via the vATPase complex, and is phosphorylated by mTORC1 on the lysosomal surface (1). This keeps TFEB inactive by cytosolic sequestration. During starvation mTORC1 is released from the LYNUS machinery and becomes inactive. Thus, TFEB can no longer be phosphorylated by mTORC1 and it translocates to the nucleus where it induces its own transcription (2). Therefore, starvation regulates TFEB activity through a dual mechanism that involves a post-translational modification (that is, phosphorylation) and a transcriptional autoregulatory loop. Once in the nucleus, TFEB regulates the expression of genes involved in the lysosomal–autophagy pathway (3). and of PGC1α–PPARα target genes (4). In this way TFEB controls the starvation response by activating both macrolipophagy (5) and fatty-acid oxidation (6). The insert shows the main components of the LYNUS machinery. The mTORC1 complex, which includes regulatory proteins associated with mTOR, such as RAPTOR, LST8, and DEPTOR, physically interacts with the RAG GTPases on the lysosomal surface and it is activated by them. A complex known as the Ragulator mediates the activation and docking of RAGS to the lysosomal membrane, and the small GTPase RHEB is also involved in the growth factor-mediated activation of mTORC1, . The vATPase complex is involved in amino acid sensing and it mediates amino acid-sensitive interactions between Rags and Ragulator, which is the initial step in lysosomal signaling. The ATP-sensitive Na+ channel lysoNaATP, which is comprised of the subunits TPC1 and TPC2, is located on the lysosomal membrane and it has recently been shown to interact with mTORC1 and participates in nutrient sensing The nature of interaction between lysoNaATP and mTORC1 is unknown but seems to be independent form other components of the LYNUS machinery and the transcription factor EB (TFEB) and its interacting proteins (see text).
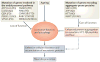
Figure 3. Defective cellular clearance in neurodegenerative diseases
Defective cellular clearance, leading to neurodegeneration, can result from two different mechanisms. First, loss-of-function mutations of genes involved in the lysosomal–-autophagic pathway (for example, ATP13A2, CATD, GBA1, PSEN1/2, VPS35, PINK, PARK, CHMP2B, RAB7, and WDR45) can affect cellular degradation and recycling processes. Second, gain-of-function mutations of aggregate-prone proteins (for example, SNCA, APP, HTT and MAPT) may lead to enhanced protein aggregation and engulfment of lysosomal–autophagic pathways. In addition, a global decrease of lysosomal–autophagy function has been observed during ageing and may contribute to an impairment of cellular clearance. Ultimately, and regardless of the mechanism involved, defective cellular clearance leads to the accumulation of neurotoxic proteins and neuronal cell death. (PD = Parkinson's disease; AD = Alzheimer's diseases; FTD = Fronto-Temporal Dementia; CMT2b = Charcot-Marie-Tooth type 2B; SENDA = Static Encephalopathy of Childhood with Neurodegeneration in Adulthood).
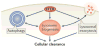
Figure 4. TFEB regulates cellular clearance
TFEB controls lysosomal biogenesis by regulating the level of lysosomal enzymes, lysosomal acidification and the number of lysosomes. TFEB also controls autophagy by regulating the number of autophagosomes and the fusion between autophagosomes and lysosomes. Finally. TFEB regulates docking and fusion of lysosomes to the plasma membrane in the process of lysosomal exocytosis. The concerted action of these three processes leads to cellular clearance. TFEB
Similar articles
- A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB.
Settembre C, Zoncu R, Medina DL, Vetrini F, Erdin S, Erdin S, Huynh T, Ferron M, Karsenty G, Vellard MC, Facchinetti V, Sabatini DM, Ballabio A. Settembre C, et al. EMBO J. 2012 Mar 7;31(5):1095-108. doi: 10.1038/emboj.2012.32. Epub 2012 Feb 17. EMBO J. 2012. PMID: 22343943 Free PMC article. - Lysosomal adaptation: how the lysosome responds to external cues.
Settembre C, Ballabio A. Settembre C, et al. Cold Spring Harb Perspect Biol. 2014 May 5;6(6):a016907. doi: 10.1101/cshperspect.a016907. Cold Spring Harb Perspect Biol. 2014. PMID: 24799353 Free PMC article. - Activation of peroxisome proliferator-activated receptor α induces lysosomal biogenesis in brain cells: implications for lysosomal storage disorders.
Ghosh A, Jana M, Modi K, Gonzalez FJ, Sims KB, Berry-Kravis E, Pahan K. Ghosh A, et al. J Biol Chem. 2015 Apr 17;290(16):10309-24. doi: 10.1074/jbc.M114.610659. Epub 2015 Mar 6. J Biol Chem. 2015. PMID: 25750174 Free PMC article. - The lysosome: from waste bag to potential therapeutic target.
Appelqvist H, Wäster P, Kågedal K, Öllinger K. Appelqvist H, et al. J Mol Cell Biol. 2013 Aug;5(4):214-26. doi: 10.1093/jmcb/mjt022. J Mol Cell Biol. 2013. PMID: 23918283 Review. - Lysosomes as dynamic regulators of cell and organismal homeostasis.
Ballabio A, Bonifacino JS. Ballabio A, et al. Nat Rev Mol Cell Biol. 2020 Feb;21(2):101-118. doi: 10.1038/s41580-019-0185-4. Epub 2019 Nov 25. Nat Rev Mol Cell Biol. 2020. PMID: 31768005 Review.
Cited by
- The lysosomal proteome of senescent cells contributes to the senescence secretome.
Rovira M, Sereda R, Pladevall-Morera D, Ramponi V, Marin I, Maus M, Madrigal-Matute J, Díaz A, García F, Muñoz J, Cuervo AM, Serrano M. Rovira M, et al. Aging Cell. 2022 Oct;21(10):e13707. doi: 10.1111/acel.13707. Epub 2022 Sep 10. Aging Cell. 2022. PMID: 36087066 Free PMC article. - Lipids, lysosomes, and autophagy.
Jaishy B, Abel ED. Jaishy B, et al. J Lipid Res. 2016 Sep;57(9):1619-35. doi: 10.1194/jlr.R067520. Epub 2016 Jun 21. J Lipid Res. 2016. PMID: 27330054 Free PMC article. Review. - Causal relationships between rheumatoid arthritis and neurodegenerative diseases: a two-sample univariable and multivariable Mendelian randomization study.
Chen X, Cai L, Fan W, Yang Q, Mao X, Yao L. Chen X, et al. Front Med (Lausanne). 2024 Aug 13;11:1439344. doi: 10.3389/fmed.2024.1439344. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39193017 Free PMC article. - Recent insights into the function of autophagy in cancer.
Amaravadi R, Kimmelman AC, White E. Amaravadi R, et al. Genes Dev. 2016 Sep 1;30(17):1913-30. doi: 10.1101/gad.287524.116. Genes Dev. 2016. PMID: 27664235 Free PMC article. Review. - Turn up the lysosome.
Saftig P, Haas A. Saftig P, et al. Nat Cell Biol. 2016 Sep 28;18(10):1025-7. doi: 10.1038/ncb3409. Nat Cell Biol. 2016. PMID: 27684505 Review.
References
- de Duve C. The lysosome turns fifty. Nature cell biology. 2005;7:847–9. - PubMed
- Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nature reviews Molecular cell biology. 2009;10:623–35. - PubMed
- Luzio JP, Parkinson MD, Gray SR, Bright NA. The delivery of endocytosed cargo to lysosomes. Biochemical Society transactions. 2009;37:1019–21. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources