Role of Janus kinase 3 in mucosal differentiation and predisposition to colitis - PubMed (original) (raw)
Role of Janus kinase 3 in mucosal differentiation and predisposition to colitis
Jayshree Mishra et al. J Biol Chem. 2013.
Abstract
Janus kinase 3 (Jak3) is a nonreceptor tyrosine kinase expressed in both hematopoietic and nonhematopoietic cells. Previously, we characterized the functions of Jak3 in cytoskeletal remodeling, epithelial wound healing, and mucosal homeostasis. However, the role of Jak3 in mucosal differentiation and inflammatory bowel disease was not known. In this report, we characterize the role of Jak3 in mucosal differentiation, basal colonic inflammation, and predisposition toward colitis. Using the Jak3 knock-out (KO) mouse model, we show that Jak3 is expressed in colonic mucosa of mice, and the loss of mucosal expression of Jak3 resulted in reduced expression of differentiation markers for the cells of both enterocytic and secretory lineages. Jak3 KO mice showed reduced expression of colonic villin, carbonic anhydrase, secretory mucin muc2, and increased basal colonic inflammation reflected by increased levels of pro-inflammatory cytokines IL-6 and IL-17A in colon along with increased colonic myeloperoxidase activity. The inflammations in KO mice were associated with shortening of colon length, reduced cecum length, decreased crypt heights, and increased severity toward dextran sulfate sodium-induced colitis. In differentiated human colonic epithelial cells, Jak3 redistributed to basolateral surfaces and interacted with adherens junction (AJ) protein β-catenin. Jak3 expression in these cells was essential for AJ localization of β-catenin and maintenance of epithelial barrier functions. Collectively, these results demonstrate the essential role of Jak3 in the colon where it facilitated mucosal differentiation by promoting the expression of differentiation markers and enhanced colonic barrier functions through AJ localization of β-catenin.
Keywords: Colitis; Differentiation; Inflammatory Bowel Disease; Innate Immunity; Jak Kinase; β-Catenin.
Figures
FIGURE 1.
Knock-out of jak3 gene leads to increased severity toward DSS-induced colitis. A, time course of body weight change during DSS-induced colitis. WT and _jak3_−/− (KO) mice (n = 6, each group) were treated with water or 2.5% DSS for the indicated periods, and the percent change in body weight was plotted versus days of treatment. B, time course of the disease activity index during DSS treatment. The disease activity index was determined in WT and KO mice as described under “Experimental Procedures” and plotted versus days of treatment. C, MPO in colonic mucosal scrapings. MPO activity was determined as described under “Experimental Procedures.” D and E, histological analysis of DSS-induced colitis. Fresh frozen colonic sections from WT and KO mice were stained with H&E; representative sections are shown with indicated magnifications from each group (n = 6) (D). E, colonic H&E sections from each animal under D were scored in a blinded fashion using protocol described under “Experimental Procedures.” F, colon to body weight ratios of DSS-treated WT (control) and Jak3 KO mice were calculated, and values are shown. A–C, E, and F, values are mean ± S.E. Asterisks indicate statistically significant differences between WT and KO groups (p < 0.05 in at least n = 3 independent experiments).
FIGURE 2.
Increased basal colonic inflammation in Jak3 KO mice. A–C, shortening of colon and cecum lengths in Jak3 KO mice. A, representative (n = 5) macroscopic images of the colons with cecum from WT and KO mice are shown. Colon (B) and cecum (C) lengths of WT and KO mice were calculated using NIS Element software (Nikon®), and mean values (n = 5) were plotted for each group. D, histological analysis of colon sections from mice treated with water. Fresh frozen colonic sections from WT and KO mice were stained with H&E; D, representative sections are shown with the indicated magnifications from each group (n = 6). Black arrows (×40) show comparison of colonic mucosa indicating perturbed epithelial architecture with interruptions in epithelial lining in KO mice. Red arrows (×100 and ×200) indicate discontinuous mucous layer overlaying epithelial mucosa. Yellow double arrows (×200) with oval lining around indicates reduced crypt lengths in KO mice. Black arrows and blue lines (×400) indicate reduced crypt width with irregular arrangement of cells from the base of the crypt to the luminal surface in KO mice. E, epidermal thickness (crypt length) from H&E-stained colonic sections (from D) of WT and KO was quantified using the NIS Element imaging software (Nikon®), and mean values from each group (n = 6 mice per group) are plotted. F, increased colonic MPO activity in untreated KO mice. MPO activities in colonic tissue lysates from untreated mice were determined, and mean values from each group (n = 6 mice per group) were plotted. G and H, increased pro-inflammatory cytokines in colon of KO mice. Cytokine level in the colonic tissue lysates from WT and KO mice were measured using a mouse MultiAnalyte cytokine assay kit (Qiagen) as per the manufacturer's protocol, and mean values from each group (n = 6 mice per group) are shown. B, C, and E–H, values are mean ± S.E. Asterisks indicate statistically significant differences between WT and KO groups (p < 0.05 from at least n = 3 independent experiments).
FIGURE 3.
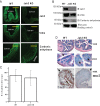
Compromised colonic mucosal differentiations in Jak3 KO mice. A, immunofluorescence staining of colonic mucosa. Colonic tissue sections from WT and KO mice were stained using indicated primary antibodies. Images were acquired using Nikon C1-plus laser confocal microscope, and representative images are shown from each group (n = 6). Note that Jak3 is localized to both crypt and villus of colonic mucosa in WT but is completely absent in KO mice. Bar, 550 μm. B, colonic tissue lysates were analyzed using IB for indicated proteins using β-actin as control. Representative blots (n = 3) are shown. C and D, mucin-secreting goblets cells were affected in Jak3 KO mice. Periodic acid-Schiff staining for goblet cells (D) in colonic tissue sections of WT and KO mice were performed, and average numbers of goblet cells per crypt were determined using NIS Element imaging software for each group (n = 6 mice per group with 5 crypts per mice) using protocol described under “Experimental Procedures.”. The mean values (C) of goblet cells per crypt are shown. Black arrows (D, middle panels) show the comparison of luminal surface-localized goblet cells. Notice that KO mice had dispersed goblet cells with less localization toward luminal surfaces. D, bottom panels, immunohistochemical staining for muc2 expression in colonic mucosa of WT and KO mice was performed using the protocol described under “Experimental Procedures.” The data shown are representative (n = 6 per group) of positive-stained areas for muc-2. Black arrows (D, bottom panels) show the absence of muc2-positive cells in KO mice.
FIGURE 4.
Jak3 promotes expression of enterocytic differentiation markers in human IEC. A, left panel, human-derived colonic epithelial cells HT-29 Cl-19A were grown until subconfluence (Sub), confluence (Conf), and 2 and 4 weeks (wks) postconfluence. Cells lysates were analyzed by IB for the indicated proteins using β-actin as control. Representative blots (n = 3) are shown. Right panel, similar experiments were performed as under the left panel except using HT-29 Cl-19a cells that were stably transfected with wild type HA-tagged Jak3. B, Jak3 expression promotes TEER. Control, HA-tagged Jak3-expressing (Jak3-HT-29) and doxycycline-regulated RFP-tagged Jak3-shRNA (shRNA-HT-29) expressing in Jak3-HT-29 cells were cultured in a 24-transwell plate to confluence, and TEER was measured. Mean values (n = 6 independent experiments) of TEER are shown. For Jak3 shRNA-HT-29, the expression of RFP-tagged Jak3-shRNA was induced by supplementing doxycycline in the growth media. Previously, we reported that this leads to knock down of Jak3 expression in HT-29 CL-19A cells where treatment with doxycyline alone in untransduced cells had no effect on Jak3 expression (25). C and D, Jak3 KO mice show symptoms of PLE. Serum total protein and albumin (C) and fecal α1-antitrypsin (D) were determined in serum and fecal extracts, respectively, from WT and KO mice using methods described under “Experimental Procedures.” Lower panel in C and D show representative images (n = 3). B–D, values are means ± S.E. (n = 6). Asterisks indicate statistically significant differences (p < 0.05) from the control cells.
FIGURE 5.
Jak3 facilitates AJ formation through interactions with β-catenin. A, Jak3 redistributes to basolateral surfaces in differentiated human IEC. Cellular redistribution of Jak3 was determined using IFM in confluent (nondifferentiated) and 2-week post-confluent (as these had maximum expression of differentiation markers, Fig. 4_A_) HT-29 Cl-19a cells. Bright field images (left panels) and corresponding XZ section (right panels) of Jak3 (green)-stained cells were taken to determine the basolateral redistribution using AJ protein β-catenin as a positive control. Representative images are shown (n = 5 experiments). B, Jak3 regulates β-catenin localization to AJ. AJ localization of β-catenin was determined in 2-week post-confluent HT-29 CL-19a cells transduced with lentiviruses mentioned in Fig. 4_B_ and grown in the presence (+Dox) or absence (−Dox) of doxycycline, and IFM was performed using antibody for β-catenin. Representative images are shown (n = 6 experiments), where the green color indicates β-catenin and the red color confirms RFP-tagged Jak3-shRNA expression-mediated knockdown of Jak3 expression (25). Note that knockdown of Jak3 expression disrupts the AJ localization of β-catenin (white arrows) as denoted by punctate green staining in Jak3-shRNA-expressing (+Dox) cells. Bar 14 μm. C, Jak3 activation facilitates its interactions with tyrosine 654-phosphorylated pool of β-catenin. Left panel, co-IP followed by IB studies were done using cell lysates from HT-29 CL-19a cells and indicated antibodies. Representative blots are shown (n = 3 experiments). C, right panel, effect of inhibition of Jak3 on the interactions between Jak3 and β-catenin was studied using co-IP followed by IB studies from lysates of HT-29 CL-19A cells treated with Jak3 inhibitor WHI-P131 (25 μ
m
). Previously, we reported that treatment with WHI-P131 leads to inhibition of Jak3 in HT-29 CL-19A cells (12, 13, 25). D, knock-out of Jak3 affects localization of β-catenin in colonic mucosa of mice. Localization of β-catenin was studied in colonic tissue sections from WT and KO mice using IFM as described in Fig. 3_A_. Representative images are shown (n = 5 experiments with three tissue sections per experiment). Profuse β-catenin staining in WT colon is evident in the crypt and villus regions (yellow and white arrows, respectively), which were absent in KO mice. Scale bar, 550 μm.
FIGURE 6.
Proposed model for the role of Jak3 in colonic mucosal health and predisposition to colitis.
Similar articles
- Janus kinase 3 regulates adherens junctions and epithelial mesenchymal transition through β-catenin.
Mishra J, Das JK, Kumar N. Mishra J, et al. J Biol Chem. 2017 Oct 6;292(40):16406-16419. doi: 10.1074/jbc.M117.811802. Epub 2017 Aug 17. J Biol Chem. 2017. PMID: 28821617 Free PMC article. - Knockout of ClC-2 reveals critical functions of adherens junctions in colonic homeostasis and tumorigenicity.
Jin Y, Ibrahim D, Magness ST, Blikslager AT. Jin Y, et al. Am J Physiol Gastrointest Liver Physiol. 2018 Dec 1;315(6):G966-G979. doi: 10.1152/ajpgi.00087.2018. Epub 2018 Oct 4. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 30285466 Free PMC article. - Intestinal breast cancer resistance protein (BCRP) requires Janus kinase 3 activity for drug efflux and barrier functions in obesity.
Mishra J, Simonsen R, Kumar N. Mishra J, et al. J Biol Chem. 2019 Nov 29;294(48):18337-18348. doi: 10.1074/jbc.RA119.007758. Epub 2019 Oct 25. J Biol Chem. 2019. PMID: 31653704 Free PMC article. - Mucosal Epithelial Jak Kinases in Health and Diseases.
Kumar N, Kuang L, Villa R, Kumar P, Mishra J. Kumar N, et al. Mediators Inflamm. 2021 Mar 16;2021:6618924. doi: 10.1155/2021/6618924. eCollection 2021. Mediators Inflamm. 2021. PMID: 33814980 Free PMC article. Review.
Cited by
- Pattern Recognition Receptor Signaling and Cytokine Networks in Microbial Defenses and Regulation of Intestinal Barriers: Implications for Inflammatory Bowel Disease.
Abraham C, Abreu MT, Turner JR. Abraham C, et al. Gastroenterology. 2022 May;162(6):1602-1616.e6. doi: 10.1053/j.gastro.2021.12.288. Epub 2022 Feb 9. Gastroenterology. 2022. PMID: 35149024 Free PMC article. Review. - Lessons Learned From Trials Targeting Cytokine Pathways in Patients With Inflammatory Bowel Diseases.
Abraham C, Dulai PS, Vermeire S, Sandborn WJ. Abraham C, et al. Gastroenterology. 2017 Feb;152(2):374-388.e4. doi: 10.1053/j.gastro.2016.10.018. Epub 2016 Oct 22. Gastroenterology. 2017. PMID: 27780712 Free PMC article. Review. - IL-22-Activated MUC13 Impacts on Colonic Barrier Function through JAK1/STAT3, SNAI1/ZEB1 and ROCK2/MAPK Signaling.
Breugelmans T, Arras W, Oosterlinck B, Jauregui-Amezaga A, Somers M, Cuypers B, Laukens K, De Man JG, De Schepper HU, De Winter BY, Smet A. Breugelmans T, et al. Cells. 2023 Apr 23;12(9):1224. doi: 10.3390/cells12091224. Cells. 2023. PMID: 37174625 Free PMC article. - Janus kinase 3 regulates adherens junctions and epithelial mesenchymal transition through β-catenin.
Mishra J, Das JK, Kumar N. Mishra J, et al. J Biol Chem. 2017 Oct 6;292(40):16406-16419. doi: 10.1074/jbc.M117.811802. Epub 2017 Aug 17. J Biol Chem. 2017. PMID: 28821617 Free PMC article. - Null Function of Npr1 Disturbs Immune Response in Colonic Inflammation During Early Postnatal Stage.
Long C, Liu H, Zhan W, Chen L, Wu A, Yang L, Chen S. Long C, et al. Inflammation. 2022 Dec;45(6):2419-2432. doi: 10.1007/s10753-022-01702-4. Epub 2022 Jul 7. Inflammation. 2022. PMID: 35794311 Free PMC article.
References
- Sancho E., Batlle E., Clevers H. (2003) Live and let die in the intestinal epithelium. Curr. Opin. Cell Biol. 15, 763–770 - PubMed
- Dignass A. U. (2001) Mechanisms and modulation of intestinal epithelial repair. Inflamm. Bowel. Dis. 7, 68–77 - PubMed
- Gersemann M., Becker S., Kübler I., Koslowski M., Wang G., Herrlinger K. R., Griger J., Fritz P., Fellermann K., Schwab M., Wehkamp J., Stange E. F. (2009) Differences in goblet cell differentiation between Crohn's disease and ulcerative colitis. Differentiation 77, 84–94 - PubMed
- Christodoulou K., Wiskin A. E., Gibson J., Tapper W., Willis C., Afzal N. A., Upstill-Goddard R., Holloway J. W., Simpson M. A., Beattie R. M., Collins A., Ennis S. (2013) Next generation exome sequencing of paediatric inflammatory bowel disease patients identifies rare and novel variants in candidate genes. Gut 62, 977–984 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous