Capsaicin-sensitive sensory nerves exert complex regulatory functions in the serum-transfer mouse model of autoimmune arthritis - PubMed (original) (raw)
Capsaicin-sensitive sensory nerves exert complex regulatory functions in the serum-transfer mouse model of autoimmune arthritis
Éva Borbély et al. Brain Behav Immun. 2015 Mar.
Abstract
Objective: The K/BxN serum-transfer arthritis is a widely-used translational mouse model of rheumatoid arthritis, in which the immunological components have thoroughly been investigated. In contrast, little is known about the role of sensory neural factors and the complexity of neuro-immune interactions. Therefore, we analyzed the involvement of capsaicin-sensitive peptidergic sensory nerves in autoantibody-induced arthritis with integrative methodology.
Methods: Arthritogenic K/BxN or control serum was injected to non-pretreated mice or resiniferatoxin (RTX)-pretreated animals where capsaicin-sensitive nerves were inactivated. Edema, touch sensitivity, noxious heat threshold, joint function, body weight and clinical arthritis severity scores were determined repeatedly throughout two weeks. Micro-CT and in vivo optical imaging to determine matrix-metalloproteinase (MMP) and neutrophil-derived myeloperoxidase (MPO) activities, semiquantitative histopathological scoring and radioimmunoassay to measure somatostatin in the joint homogenates were also performed.
Results: In RTX-pretreated mice, the autoantibody-induced joint swelling, arthritis severity score, MMP and MPO activities, as well as histopathological alterations were significantly greater compared to non-pretreated animals. Self-control quantification of the bone mass revealed decreased values in intact female mice, but significantly greater arthritis-induced pathological bone formation after RTX-pretreatment. In contrast, mechanical hyperalgesia from day 10 was smaller after inactivating capsaicin-sensitive afferents. Although thermal hyperalgesia did not develop, noxious heat threshold was significantly higher following RTX pretreatment. Somatostatin-like immunoreactivity elevated in the tibiotarsal joints in non-pretreated, which was significantly less in RTX-pretreated mice.
Conclusions: Although capsaicin-sensitive sensory nerves mediate mechanical hyperalgesia in the later phase of autoantibody-induced chronic arthritis, they play important anti-inflammatory roles at least partially through somatostatin release.
Keywords: Capsaicin-sensitive sensory nerves; Inflammation; Matrix-metalloproteinase; Pain; Somatostatin.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Supplementary Fig. 1
Serum-induced weight loss and impaired joint function: data points represent the percentage change of weight loss of male (A) and female (B) mice compared to the initial control values and the absolute values of time spent on the grid for male (C) and female animals (D) (n = 4–5/control groups, n = 6–8/arthritic groups; two-way ANOVA + Bonferroni’s modified _t_-test).
Fig. 1
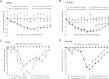
Serum-induced edema and arthritis score throughout the 2 weeks experimental period. Data points represent the percentage increase of the paw volume of male (A) and female (B) mice compared to the initial control values and the absolute values of arthritis scores for male (C) and female animals (D) (n = 4–5/non-inflamed groups, n = 6–8/arthritic groups; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 vs. non-pretreated; two-way ANOVA + Bonferroni’s modified _t_-test).
Fig. 2
Serum-induced hyperalgesia throughout the 2 weeks experimental period. Data points represent the percentage decrease of mechanonociceptive threshold for male (A) and female (B) mice, and the absolute values of noxious heat threshold for male (C) and female animals (D) (n = 4–5/control non-inflamed groups, n = 6–8/arthritic groups; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 vs. non-pretreated; two-way ANOVA + Bonferroni’s modified _t_-test).
Fig. 3
Change of neutrophil-derived myeloperoxidase activity. Panel (A) shows representative pretreatment control images, whereas panels (B and C) demonstrate inflammatory neutrophil activity on day 2 and 6 following arthritis induction, respectively. (D) Quantification of luminol bioluminescence in the diseased ankle joints. (n = 6–8 male mice/group, ###p < 0.001 vs. controls, ∗∗∗p < 0.001 vs. non-pretreated; Student _t_-test).
Fig. 4
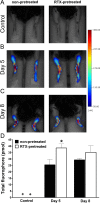
Matrix-metalloproteinase activity in the diseased hind limbs. (A) Representative pretreatment control images, (B and C) demonstrate inflammatory matrix-metalloproteinase activity on day 5 and 8 following arthritis induction. (D) Quantification the amount of fluorophore in the inflamed ankle joints (n = 3–4 male mice/group, ∗p < 0.05 vs. non-pretreated, + indicates that the probe was tested in intact mice in a self-control manner before the induction of the inflammation, but remained below the detection threshold; Student _t_-test).
Fig. 5
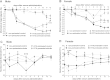
Bone structural changes in the inflamed region. (A) Representative micro-CT images of the same mice, in intact state and on day 14. (B and C) Bone volume/total volume ratio in the ankle joint, expressed as raw data and as percentage of the initial self-controls. (D and E) Bone volume/total volume ratio in the distal tibia, expressed as raw data and as percentage of the initial self-controls (n = 6/group, #p < 0.05, ##p < 0.01, ###p < 0.001 vs. controls, ∗∗p < 0.01, ∗∗∗p < 0.001 vs. non-pretreated; two-way ANOVA + Dunnett and Tukey post-tests).
Fig. 6
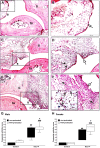
Histopathological changes of the ankle joints. Panels (A and B) show representative histopathological pictures of an intact tibiotarsal joint (ti: tibia, ta: tarsus, s: synovium), panels (C and D) demonstrate the joint structure of a non-pretreated mouse on day 14 after arthritogenic serum administration, panels (E and F) show the significantly pronounced arthritic changes of RTX-pretreated animals. (G and H) Semiquantitative histopathological scoring on the basis of synovial enlargement (white arrows), inflammatory cell accumulation (black arrows), fibroblast formation with collagen deposition (two headed arrows). Box plots represent the composite scores for male and female animals (n = 4–5/control non-inflamed groups, n = 6–8/arthritic groups; ##p < 0.01, ###p < 0.001 vs. controls, ∗p < 0.05 vs. non-pretreated; Kruskal–Wallis followed by Dunn’s post-test).
Similar articles
- Regulatory role of capsaicin-sensitive peptidergic sensory nerves in the proteoglycan-induced autoimmune arthritis model of the mouse.
Horváth Á, Borbély É, Bölcskei K, Szentes N, Kiss T, Belák M, Rauch T, Glant T, Zákány R, Juhász T, Karanyicz E, Boldizsár F, Helyes Z, Botz B. Horváth Á, et al. J Neuroinflammation. 2018 Dec 3;15(1):335. doi: 10.1186/s12974-018-1364-5. J Neuroinflammation. 2018. PMID: 30509328 Free PMC article. - Capsaicin-Sensitive Peptidergic Sensory Nerves Are Anti-Inflammatory Gatekeepers in the Hyperacute Phase of a Mouse Rheumatoid Arthritis Model.
Botz B, Kriszta G, Bölcskei K, Horváth ÁI, Mócsai A, Helyes Z. Botz B, et al. Int J Mol Sci. 2021 Feb 8;22(4):1682. doi: 10.3390/ijms22041682. Int J Mol Sci. 2021. PMID: 33567493 Free PMC article. - Complex Role of Capsaicin-Sensitive Afferents in the Collagen Antibody-Induced Autoimmune Arthritis of the Mouse.
Borbély É, Kiss T, Szabadfi K, Pintér E, Szolcsányi J, Helyes Z, Botz B. Borbély É, et al. Sci Rep. 2018 Oct 29;8(1):15916. doi: 10.1038/s41598-018-34005-6. Sci Rep. 2018. PMID: 30374145 Free PMC article. - Differential effects of TRPV channel block on polymodal activation of rat cutaneous nociceptors in vitro.
St Pierre M, Reeh PW, Zimmermann K. St Pierre M, et al. Exp Brain Res. 2009 Jun;196(1):31-44. doi: 10.1007/s00221-009-1808-3. Epub 2009 Apr 30. Exp Brain Res. 2009. PMID: 19404626 Review. - Capsaicin-sensitive afferent sensory nerves in modulating gastric mucosal defense against noxious agents.
Abdel-Salam OM, Debreceni A, Mózsik G, Szolcsányi J. Abdel-Salam OM, et al. J Physiol Paris. 1999 Nov;93(5):443-54. doi: 10.1016/s0928-4257(99)00115-1. J Physiol Paris. 1999. PMID: 10674923 Review.
Cited by
- The potential role of TRPV1 in pulmonary hypertension: Angel or demon?
Zhang X, Ye L, Huang Y, Ding X, Wang L. Zhang X, et al. Channels (Austin). 2019 Dec;13(1):235-246. doi: 10.1080/19336950.2019.1631106. Channels (Austin). 2019. PMID: 31189399 Free PMC article. Review. - Regulatory role of capsaicin-sensitive peptidergic sensory nerves in the proteoglycan-induced autoimmune arthritis model of the mouse.
Horváth Á, Borbély É, Bölcskei K, Szentes N, Kiss T, Belák M, Rauch T, Glant T, Zákány R, Juhász T, Karanyicz E, Boldizsár F, Helyes Z, Botz B. Horváth Á, et al. J Neuroinflammation. 2018 Dec 3;15(1):335. doi: 10.1186/s12974-018-1364-5. J Neuroinflammation. 2018. PMID: 30509328 Free PMC article. - The Regulation of Immunological Processes by Peripheral Neurons in Homeostasis and Disease.
Ordovas-Montanes J, Rakoff-Nahoum S, Huang S, Riol-Blanco L, Barreiro O, von Andrian UH. Ordovas-Montanes J, et al. Trends Immunol. 2015 Oct;36(10):578-604. doi: 10.1016/j.it.2015.08.007. Trends Immunol. 2015. PMID: 26431937 Free PMC article. Review. - Role of Transient Receptor Potential Ankyrin 1 Ion Channel and Somatostatin sst4 Receptor in the Antinociceptive and Anti-inflammatory Effects of Sodium Polysulfide and Dimethyl Trisulfide.
Bátai IZ, Horváth Á, Pintér E, Helyes Z, Pozsgai G. Bátai IZ, et al. Front Endocrinol (Lausanne). 2018 Feb 27;9:55. doi: 10.3389/fendo.2018.00055. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29535682 Free PMC article. - Osteosarcoma-Induced Pain Is Mediated by Glial Cell Activation in the Spinal Dorsal Horn, but Not Capsaicin-Sensitive Nociceptive Neurons: A Complex Functional and Morphological Characterization in Mice.
Bencze N, Scheich B, Szőke É, Wilhelm I, Körmöndi S, Botz B, Helyes Z. Bencze N, et al. Cancers (Basel). 2024 May 7;16(10):1788. doi: 10.3390/cancers16101788. Cancers (Basel). 2024. PMID: 38791867 Free PMC article.
References
- Alarcón G.S. Methotrexate use in rheumatoid arthritis. A Clinician’s perspective. Immunopharmacology. 2000;47:259–271. - PubMed
- Anichini M., Cesaretti S., Lepori M., Maddali Bongi S., Maresca M., Zoppi M. Substance P in the serum of patients with rheumatoid arthritis. Rev. Rhum. Engl. Ed. 1997;64:18–21. - PubMed
- Arnhold J., Flemmig J. Human myeloperoxidase in innate and acquired immunity. Arch. Biochem. Biophys. 2010;500:92–106. - PubMed
- Bevaart L., Vervoordeldonk M.J., Tak P.P. Evaluation of therapeutic targets in animal models of arthritis: how does it relate to rheumatoid arthritis? Arthritis Rheum. 2010;62:2192–2205. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous