The sleep-promoting and hypothermic effects of glycine are mediated by NMDA receptors in the suprachiasmatic nucleus - PubMed (original) (raw)
The sleep-promoting and hypothermic effects of glycine are mediated by NMDA receptors in the suprachiasmatic nucleus
Nobuhiro Kawai et al. Neuropsychopharmacology. 2015 May.
Abstract
The use of glycine as a therapeutic option for improving sleep quality is a novel and safe approach. However, despite clinical evidence of its efficacy, the details of its mechanism remain poorly understood. In this study, we investigated the site of action and sleep-promoting mechanisms of glycine in rats. In acute sleep disturbance, oral administration of glycine-induced non-rapid eye movement (REM) sleep and shortened NREM sleep latency with a simultaneous decrease in core temperature. Oral and intracerebroventricular injection of glycine elevated cutaneous blood flow (CBF) at the plantar surface in a dose-dependent manner, resulting in heat loss. Pretreatment with N-methyl-D-aspartate (NMDA) receptor antagonists AP5 and CGP78608 but not the glycine receptor antagonist strychnine inhibited the CBF increase caused by glycine injection into the brain. Induction of c-Fos expression was observed in the hypothalamic nuclei, including the medial preoptic area (MPO) and the suprachiasmatic nucleus (SCN) shell after glycine administration. Bilateral microinjection of glycine into the SCN elevated CBF in a dose-dependent manner, whereas no effect was observed when glycine was injected into the MPO and dorsal subparaventricular zone. In addition, microinjection of D-serine into the SCN also increased CBF, whereas these effects were blocked in the presence of L-701324. SCN ablation completely abolished the sleep-promoting and hypothermic effects of glycine. These data suggest that exogenous glycine promotes sleep via peripheral vasodilatation through the activation of NMDA receptors in the SCN shell.
Figures
Figure 1
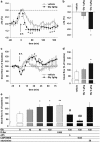
The effects of glycine on core temperature and sleep in a new cage environment. Core body temperature (a) and locomotor activity (b) were recorded from 60 min before cage exchange. All values are the mean±SEM (_N_=7). (c) Wake (left), NREM (middle), and REM (right) sleep amount changes are shown as the percentage of time spent in each stage every 30 min after oral administration of vehicle and 2 g/kg glycine (_N_=7 each). Amount (d), total episode number (e), and mean duration (f) of each stage during the first 90 min after oral administration. Glycine treatment significantly increased NREM sleep compared with vehicle during the first 90 min after administration, but it had no effect on all parameters after 90 min. Asterisks indicate significant difference (*p<0.05, **p<0.01). (g) NREM sleep latency was defined as the time from oral administration to the first appearance of a NREM sleep episode lasting longer than 1 min. (h) The distribution of EEG power during NREM and REM sleep. The data include all artifact-free sleep epochs during the first 2 h after oral administration. (i) Glycine administration significantly decreased core body temperature compared with vehicle.
Figure 2
Dose-dependent decreases in core body temperature and increases in skin blood flow following peripheral and central administration of glycine. (a) The changes in rat core body temperature (Δcore body temperature) were recorded from 60 min before administration. Each symbol represents the mean±SEM (_N_=8). (b) The difference in Δcore body temperature 30 min after oral administration between the vehicle treatment group and the 1- or 2-g/kg glycine treatment group. The data are presented as the mean±SEM. *p<0.05 values are based on comparisons with the vehicle treatment group. (c) Changes in cutaneous blood flow (CBF) were recorded from 15 min before oral administration. Each symbol represents the mean±SEM (_N_=8–10). (d) The ratio of CBF 30–45 min after oral administration to 0–15 min before administration (baseline). (e) The effect of intracerebroventricular administration of glycine and glycine-related compounds on CBF. The data are presented as the mean±SEM (_N_=5–10). *p<0.05 values are based on comparisons with baseline. #p<0.05, ##p<0.01 values are based on comparisons with the absence of inhibitors of NMDA receptors or glycine receptors.
Figure 3
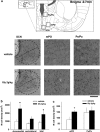
Oral administration of glycine induces c-Fos expression in the hypothalamic nuclei. (a) Typical images of c-Fos immunoreactive cells in each hypothalamic area. (b) The density of c-Fos immunoreactive cells in the SCN. (c) The density of c-Fos immunoreactive cells in Pe/Pa and mPO. The asterisks indicate a significant difference between the vehicle-treated group and the glycine-treated group (*p<0.05).
Figure 4
The microinjection of glycine into the thermoregulatory areas in the presence or absence of the specific antagonist of NMDA receptors. (a) The bilateral injection of glycine into the thermoregulatory areas. (b) The bilateral injection of glycine into the suprachiasmatic nucleus in the presence or absence of L-701324, a specific antagonist of NMDA receptors. Each column represents the mean±SEM (_n_=4–6). The asterisks indicate a significant difference between CBF 0–15 min before glycine treatment and CBF 30–45 min after glycine treatment (*p<0.05, **p<0.01). # indicates a significant difference between the presence and absence of L-701324. (#p<0.05).
Figure 5
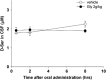
The concentration of D-serine in CSF after oral administration of glycine. Concentration-time curves of D-serine in CSF following oral administration of vehicle (empty circles) or 2 g/kg of glycine (filled circles). Each symbol represents the mean±SEM (_n_=5).
Figure 6
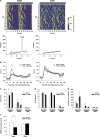
The evaluation of the effects of glycine on sleep in SCNx rats. (a) Representative temperature records (upper) and χ2 periodograms (lower) of sham (left) and SCNx (right) rats. Circadian tau was given for significant peaks (p<0.001). (b) The core body temperature was recorded from 60 min before oral administration in sham (left) and SCNx (right) rats. All values are the mean±SEM (_n_=6 for sham rats, _n_=11 for SCNx rats). The asterisks indicate significant differences (*p<0.05). Amount (c), total episode number (d), and mean duration (e) of each stage in sham and SCNx rats during the first 90 min after oral administration. (f) NREM sleep latency was not shortened by glycine administration in SCNx rats.
Similar articles
- Fos-immunoreactivity in the hypothalamus: dependency on the diurnal rhythm, sleep, gender, and estrogen.
Peterfi Z, Churchill L, Hajdu I, Obal F Jr, Krueger JM, Parducz A. Peterfi Z, et al. Neuroscience. 2004;124(3):695-707. doi: 10.1016/j.neuroscience.2003.10.047. Neuroscience. 2004. PMID: 14980739 - State-dependent pattern of Fos protein expression in regionally-specific sites within the preoptic area of the cat.
Torterolo P, Benedetto L, Lagos P, Sampogna S, Chase MH. Torterolo P, et al. Brain Res. 2009 Apr 24;1267:44-56. doi: 10.1016/j.brainres.2009.02.054. Epub 2009 Mar 6. Brain Res. 2009. PMID: 19269274 - GABA transporter-1 inhibitor NO-711 alters the EEG power spectra and enhances non-rapid eye movement sleep during the active phase in mice.
Xu XH, Qiu MH, Dong H, Qu WM, Urade Y, Huang ZL. Xu XH, et al. Eur Neuropsychopharmacol. 2014 Apr;24(4):585-94. doi: 10.1016/j.euroneuro.2013.09.002. Epub 2013 Sep 12. Eur Neuropsychopharmacol. 2014. PMID: 24080505 - New therapeutic strategy for amino acid medicine: glycine improves the quality of sleep.
Bannai M, Kawai N. Bannai M, et al. J Pharmacol Sci. 2012;118(2):145-8. doi: 10.1254/jphs.11r04fm. Epub 2012 Jan 27. J Pharmacol Sci. 2012. PMID: 22293292 Review. - Body temperature and sleep.
Szymusiak R. Szymusiak R. Handb Clin Neurol. 2018;156:341-351. doi: 10.1016/B978-0-444-63912-7.00020-5. Handb Clin Neurol. 2018. PMID: 30454599 Review.
Cited by
- Sake yeast induces the sleep-promoting effects under the stress-induced acute insomnia in mice.
Nishimon S, Sakai N, Nishino S. Nishimon S, et al. Sci Rep. 2021 Oct 21;11(1):20816. doi: 10.1038/s41598-021-00271-0. Sci Rep. 2021. PMID: 34675261 Free PMC article. - GABALAGEN Facilitates Pentobarbital-Induced Sleep by Modulating the Serotonergic System in Rats.
Ye M, Rheu KM, Lee BJ, Shim I. Ye M, et al. Curr Issues Mol Biol. 2024 Oct 4;46(10):11176-11189. doi: 10.3390/cimb46100663. Curr Issues Mol Biol. 2024. PMID: 39451543 Free PMC article. - Hypothalamic Neurochemical Changes in Long-Term Recovered Bilateral Subdiaphragmatic Vagotomized Rats.
Kobrzycka AT, Stankiewicz AM, Goscik J, Gora M, Burzynska B, Iwanicka-Nowicka R, Pierzchala-Koziec K, Wieczorek M. Kobrzycka AT, et al. Front Behav Neurosci. 2022 Jul 8;16:869526. doi: 10.3389/fnbeh.2022.869526. eCollection 2022. Front Behav Neurosci. 2022. PMID: 35874650 Free PMC article. - Sleep Basic Research on Verifying the Effects of Natural Compounds on Wakefulness or Sleep.
Nishimon S. Nishimon S. Juntendo Iji Zasshi. 2022 Feb 16;68(2):115-119. doi: 10.14789/jmj.JMJ21-0022-R. eCollection 2022. Juntendo Iji Zasshi. 2022. PMID: 38912281 Free PMC article. Review. - Whole-brain irradiation differentially modifies neurotransmitters levels and receptors in the hypothalamus and the prefrontal cortex.
Franco-Pérez J, Montes S, Sánchez-Hernández J, Ballesteros-Zebadúa P. Franco-Pérez J, et al. Radiat Oncol. 2020 Nov 23;15(1):269. doi: 10.1186/s13014-020-01716-y. Radiat Oncol. 2020. PMID: 33228731 Free PMC article.
References
- Alam MN, McGinty D, Szymusiak R. Neuronal discharge of preoptic/anterior hypothalamic thermosensitive neurons: relation to NREM sleep. Am J Physiol. 1995;269 (5 Pt 2:R1240–R1249. - PubMed
- Alfoldi P, Rubicsek G, Cserni G, Obal F., Jr. Brain and core temperatures and peripheral vasomotion during sleep and wakefulness at various ambient temperatures in the rat. Pflugers Arch. 1990;417:336–341. - PubMed
- Barrett J, Lack L, Morris M. The sleep-evoked decrease of body temperature. Sleep. 1993;16:93–99. - PubMed
- Campbell SS, Broughton RJ. Rapid decline in body temperature before sleep: fluffing the physiological pillow. Chronobiol Int. 1994;11:126–131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous