Novel κ-opioid receptor agonist MB-1C-OH produces potent analgesia with less depression and sedation - PubMed (original) (raw)
doi: 10.1038/aps.2014.145. Epub 2015 Mar 30.
Jun Wang 2, Jian-chun Chen 3, Yi-min Tao 1, Yu-hua Wang 4, Xue-jun Xu 1, Jie Chen 1, Yun-gen Xu 5, Tao Xi 5, Xiao-wu Hu 3, Yu-jun Wang 1, Jing-gen Liu 1
Affiliations
- PMID: 25816912
- PMCID: PMC4422940
- DOI: 10.1038/aps.2014.145
Novel κ-opioid receptor agonist MB-1C-OH produces potent analgesia with less depression and sedation
Le-sha Zhang et al. Acta Pharmacol Sin. 2015 May.
Abstract
Aim: To characterize the pharmacological profiles of a novel κ-opioid receptor agonist MB-1C-OH.
Methods: [(3)H]diprenorphine binding and [(35)S]GTPγS binding assays were performed to determine the agonistic properties of MB-1C-OH. Hot plate, tail flick, acetic acid-induced writhing, and formalin tests were conducted in mice to evaluate the antinociceptive actions. Forced swimming and rotarod tests of mice were used to assess the sedation and depression actions.
Results: In [(3)H]diprenorphine binding assay, MB-1C-OH did not bind to μ- and δ-opioid receptors at the concentration of 100 μmol/L, but showed a high affinity for κ-opioid receptor (Ki=35 nmol/L). In [(35)S]GTPγS binding assay, the compound had an Emax of 98% and an EC50 of 16.7 nmol/L for κ-opioid receptor. Subcutaneous injection of MB-1C-OH had no effects in both hot plate and tail flick tests, but produced potent antinociception in the acetic acid-induced writhing test (ED50=0.39 mg/kg), which was antagonized by pretreatment with a selective κ-opioid receptor antagonist Nor-BNI. In the formalin test, subcutaneous injection of MB-1C-OH did not affect the flinching behavior in the first phase, but significantly inhibited that in the second phase (ED50=0.87 mg/kg). In addition, the sedation or depression actions of MB-1C-OH were about 3-fold weaker than those of the classical κ agonist (-)U50,488H.
Conclusion: MB-1C-OH is a novel κ-opioid receptor agonist that produces potent antinociception causing less sedation and depression.
Figures
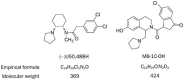
Figure 1
Chemical structures of (−)U50,488H and MB-1C-OH.
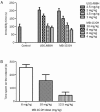
Figure 2
Effects of pretreatment with nor-BNI, β-FNA, and naltrindole on MB-1C-OH-induced antinociception in the acetic acid-induced writhing assay. Mice were pretreated with the μ-opioid receptor antagonist, β-FNA (10 mg/kg, sc) 24 h before MB-1C-OH administration; the δ-opioid receptor antagonist, naltrindole (3.0 mg/kg, sc) 30 min before MB-1C-OH administration; or the selective κ-opioid receptor antagonist, nor-BNI (10 mg/kg, sc) 15 min before MB-1C-OH administration and then injected with MB-1C-OH (1.88 mg/kg, sc). Acetic acid solution was intraperitoneally injected 15 min after drug administration. The maximum possible effect (% MPE) was calculated as described in the Materials and methods section, and the number of times the mouse writhed is also shown. Data are presented as the mean±SEM from 10 animals. c_P_<0.01 compared with the control group.
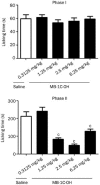
Figure 3
MB-1C-OH-induced antinociceptive effects in the formalin test. The animals were pretreated with MB-1C-OH into subcutaneous. After 15 min, the animals were injected with formalin (20 μL/paw). The amount of time the mice spent licking or flinching during first phase and second phase was recorded. All data are expressed as the mean±SEM (at least five animals in each group). c_P_<0.01 compared with the vehicle group (one-way ANOVA with Dunnett's test).
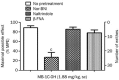
Figure 4
The depressive effect of MB-1C-OH in the forced swimming test and the sedative effect of MB-1C-OH in the rotarod test. (A) Mice were administered (sc) saline, (−)U50,488H or MB-1C-OH. After 15 min, they were put singly into transparent Plexiglas cylinders containing water at a depth of 10 cm for 6 min. The immobility time was recorded during the final 4 min. Data for each group are presented as the mean±SEM from 5–12 animals. (B) Mice were subcutaneously administered various doses of MB-1C-OH. Then, 15 min later, mice were put singly on a rotarod for 300 s. The duration time before each mouse fell off the rotarod was recorded. Data for each group are presented as the mean±SEM from 10 animals.
Similar articles
- LPK-26, a novel kappa-opioid receptor agonist with potent antinociceptive effects and low dependence potential.
Tao YM, Li QL, Zhang CF, Xu XJ, Chen J, Ju YW, Chi ZQ, Long YQ, Liu JG. Tao YM, et al. Eur J Pharmacol. 2008 Apr 28;584(2-3):306-11. doi: 10.1016/j.ejphar.2008.02.028. Epub 2008 Feb 19. Eur J Pharmacol. 2008. PMID: 18353307 - MGM-9 [(E)-methyl 2-(3-ethyl-7a,12a-(epoxyethanoxy)-9-fluoro-1,2,3,4,6,7,12,12b-octahydro-8-methoxyindolo[2,3-a]quinolizin-2-yl)-3-methoxyacrylate], a derivative of the indole alkaloid mitragynine: a novel dual-acting mu- and kappa-opioid agonist with potent antinociceptive and weak rewarding effects in mice.
Matsumoto K, Takayama H, Narita M, Nakamura A, Suzuki M, Suzuki T, Murayama T, Wongseripipatana S, Misawa K, Kitajima M, Tashima K, Horie S. Matsumoto K, et al. Neuropharmacology. 2008 Aug;55(2):154-65. doi: 10.1016/j.neuropharm.2008.05.003. Epub 2008 May 9. Neuropharmacology. 2008. PMID: 18550129 - Morphine can produce analgesia via spinal kappa opioid receptors in the absence of mu opioid receptors.
Yamada H, Shimoyama N, Sora I, Uhl GR, Fukuda Y, Moriya H, Shimoyama M. Yamada H, et al. Brain Res. 2006 Apr 14;1083(1):61-9. doi: 10.1016/j.brainres.2006.01.095. Epub 2006 Mar 10. Brain Res. 2006. PMID: 16530171 - The opioid receptor independent actions of kappa receptor agonists in the cardiovascular system.
Pugsley MK. Pugsley MK. Curr Pharm Des. 2004;10(20):2429-44. doi: 10.2174/1381612043383917. Curr Pharm Des. 2004. PMID: 15320754 Review. - Biased Opioid Receptor Ligands: Gain without Pain.
Ranjan R, Pandey S, Shukla AK. Ranjan R, et al. Trends Endocrinol Metab. 2017 Apr;28(4):247-249. doi: 10.1016/j.tem.2017.01.001. Epub 2017 Jan 16. Trends Endocrinol Metab. 2017. PMID: 28110810 Review.
Cited by
- The Kappa Opioid Receptor: A Promising Therapeutic Target for Multiple Pathologies.
Dalefield ML, Scouller B, Bibi R, Kivell BM. Dalefield ML, et al. Front Pharmacol. 2022 Jun 20;13:837671. doi: 10.3389/fphar.2022.837671. eCollection 2022. Front Pharmacol. 2022. PMID: 35795569 Free PMC article. Review. - The analgesic and anti-inflammatory effects of Salvinorin A analogue β-tetrahydropyran Salvinorin B in mice.
Paton KF, Kumar N, Crowley RS, Harper JL, Prisinzano TE, Kivell BM. Paton KF, et al. Eur J Pain. 2017 Jul;21(6):1039-1050. doi: 10.1002/ejp.1002. Epub 2017 Feb 3. Eur J Pain. 2017. PMID: 28158929 Free PMC article. - Behavioral Effects and Analgesic Profile of Hemoglobin-Derived Valorphin and Its Synthetic Analog in Rodents.
Todorov P, Assenov B, Angelov D, Dzhambazova E, Pechlivanova D. Todorov P, et al. Biomedicines. 2023 Oct 13;11(10):2783. doi: 10.3390/biomedicines11102783. Biomedicines. 2023. PMID: 37893157 Free PMC article. - Biological activity and characterization of leaf and seed lectins from Terminalia brownii: Insights into their analgesic and antiulcer properties.
Idries AH, Naser EH, Dafalla MB, Elmubarak SAA, Abdelrahim YE, Abdalrhman EA, Alwali SM, Ahmed BM, Yousef BA, Ebrahim RMA, Abdellatif AO, Awadallah AKE, Osman MEM, Konozy EHE. Idries AH, et al. Heliyon. 2024 Oct 18;10(20):e39351. doi: 10.1016/j.heliyon.2024.e39351. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39498066 Free PMC article. - Characterization of Sigma 1 Receptor Antagonist CM-304 and Its Analog, AZ-66: Novel Therapeutics Against Allodynia and Induced Pain.
Cirino TJ, Eans SO, Medina JM, Wilson LL, Mottinelli M, Intagliata S, McCurdy CR, McLaughlin JP. Cirino TJ, et al. Front Pharmacol. 2019 Jun 14;10:678. doi: 10.3389/fphar.2019.00678. eCollection 2019. Front Pharmacol. 2019. PMID: 31258480 Free PMC article.
References
- Trescot AM, Datta S, Lee M, Hansen H. Opioid pharmacology. Pain Physician. 2008;11:S133–53. - PubMed
- Ballantyne JC. Opioid analgesia: perspectives on right use and utility. Pain Physician. 2007;10:479–91. - PubMed
- Clark JA, Pasternak GW. U50,488: a kappa-selective agent with poor affinity for mu1 opiate binding sites. Neuropharmacology. 1988;27:331–2. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous