Neutron Crystal Structure of RAS GTPase Puts in Question the Protonation State of the GTP γ-Phosphate - PubMed (original) (raw)
Neutron Crystal Structure of RAS GTPase Puts in Question the Protonation State of the GTP γ-Phosphate
Ryan Knihtila et al. J Biol Chem. 2015.
Abstract
RAS GTPase is a prototype for nucleotide-binding proteins that function by cycling between GTP and GDP, with hydrogen atoms playing an important role in the GTP hydrolysis mechanism. It is one of the most well studied proteins in the superfamily of small GTPases, which has representatives in a wide range of cellular functions. These proteins share a GTP-binding pocket with highly conserved motifs that promote hydrolysis to GDP. The neutron crystal structure of RAS presented here strongly supports a protonated γ-phosphate at physiological pH. This counters the notion that the phosphate groups of GTP are fully deprotonated at the start of the hydrolysis reaction, which has colored the interpretation of experimental and computational data in studies of the hydrolysis mechanism. The neutron crystal structure presented here puts in question our understanding of the pre-catalytic state associated with the hydrolysis reaction central to the function of RAS and other GTPases.
Keywords: Ras protein; enzyme catalysis; neutron diffraction; small GTPase; structural biology.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
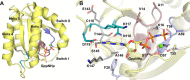
FIGURE 1.
Conserved motifs in the nucleotide-binding pocket of RAS bound to GppNHp. A, overall structure of RAS (yellow) showing its architectural features and the nucleotide-binding pocket near switch I and switch II. The nucleotide-binding motifs are colored as described below. B, nucleotide-binding pocket highlighting the motifs conserved in the superfamily of GTPases. In both A and B the P-loop or G_XXXX_GK(S/T) motif is in mauve (residues 10–17 in RAS, GAGGVGKS), the D_XX_G motif is in _purpl_e (residues 57–60 in RAS, DTAG), the NK_X_D motif is in teal (residues 116–119 in RAS,NKCD), and the E_X_SAK motif is in gray (residues 143–147 in RAS, ETSAK). Conserved switch I residues Phe-28 and Thr-35 are in light gray. The side chain H-bonding interactions within 3.2 Å as well as interactions with the Mg2+ ion are shown by black dashed lines.
FIGURE 2.
Nucleotide GppNHp and the magnesium ion in their respective binding sites on RAS. The neutron structure is shown in yellow with nuclear density in blue. The x-ray structure is in pink with electron density in gray. In the neutron crystal structure, hydrogen atoms are shown in light gray and deuterium atoms in light green. No hydrogen atoms are shown in the x-ray crystal structure. A, GppNHp from the neutron crystal structure with 2_Fo_ − Fc nuclear density contoured at the 1σ level. Note the presence of robust density at deuterium positions and breaks in the density for hydrogen atoms. B, GppNHp from the x-ray crystal structure with 2_Fo_ − Fc electron density contoured at the 1σ level. The GppNHp molecule from the neutron structure (yellow) is superimposed on that of the x-ray structure (pink). For clarity, density is shown only for the nucleotide in A and B. C, Mg2+ ion (green) with its coordination sphere on RAS and nuclear density contoured at the 1.5σ level. D, Mg2+ ion (green) with its coordination sphere on RAS and electron density contoured at the 1.5σ level. H-bonding and electrostatic interactions within 3.2 Å are shown by black dashed lines.
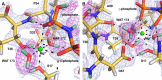
FIGURE 3.
γ-Phosphate of GppNHp and its interactions with RAS. The 2_Fo_ − Fc nuclear density map contoured at the 1.5σ level is shown in blue. A, protonated γ-phosphate and nucleophilic water molecule. Note the clear density supporting the deuterium atom on the γ-phosphate group, whereas density is only present for the oxygen atoms of the nucleophilic water molecule 175 and its close neighbor water molecule 433. B, Lys-16 interacts with both the γ- and β-phosphate groups of GppNHp and stabilizes the P-loop through its interactions with the carbonyl groups of Gly-10 and Ala-11. Atoms are color-coded in this and all subsequent figures containing models from the neutron diffraction data as follows: carbon, yellow; nitrogen, blue; oxygen, red; phosphorus, orange; hydrogen, light gray; deuterium, light green, and Mg2+, green. H-bonding interactions within 3.2 Å as well as the interaction with the Mg2+ ion are shown by black dashed lines.
FIGURE 4.
Mg2+ ion and its interactions with RAS. The 2_Fo_ − Fc nuclear density map contoured at the 1.5σ level is shown in blue, and the composite D-Omit Fo − Fc nuclear density map for active site deuterium atoms contoured at the 2.5σ level is shown in magenta. A, Mg2+ ion coordinates two oxygen atoms of the nucleotide, two protein atoms (the hydroxyl groups of Ser-17 and Thr-35), and two water molecules (172 and 173). Asp-33 is shown in the back, and its carbonyl group accepts an H-bond from Wat-172, helping to polarize it for coordinating the Mg2+. B, side chain of Asp-57 serves a similar function, accepting H-bonds from both Wat-173 and Ser-17, both of which interact directly with the Mg2+ ion. H-bonding interactions within 3.2 Å as well as the interactions with the Mg2+ ion are shown by black dashed lines.
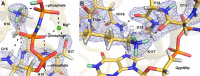
FIGURE 5.
P-loop interactions with the phosphate groups of the nucleotide. A, ring of amides, consisting of residues 15–18, interacts closely with the α- and β-phosphates of GppNHp on RAS. Nuclear density is shown in blue at the 1.5σ level for the P-loop residues only, with other density omitted for clarity. Note the lack of nuclear density for hydrogen atoms in the backbone amide groups, indicating that they have not been exchanged for deuterium. The Lys-16 side chain amino group hydrogen atoms have clearly been exchanged to deuterium and interact with the β- and γ-phosphate groups of the nucleotide. B, Gly-13 and Val-14 of the P-loop with nuclear density for the backbone amides indicating H/D exchange. This view also shows the connection between the P-loop and the NKCD (Asn-116 and Lys-117) and ETSAK (Thr-144) motifs through the backbone carbonyl groups of Gly-13 and Val-14. H-bonding interactions within 3.2 Å as well as the interaction with the Mg2+ ion are shown by black dashed lines.
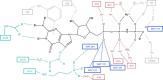
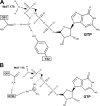
FIGURE 6.
Schematic diagram of the GppNHp interactions in the active site of RAS. The nucleotide is in black, and the protein residues are colored as in Fig. 1 according to the conserved motifs to which they belong. Water molecules are in blue. Deuterium atoms visible in the neutron density maps are shown in bold. Note that only residues in the P-loop (mauve), switch I residues (light gray), and residues in the NKCD motif (teal) interact directly with the nucleotide. Residues in the DTAG motif (including Asp-57) and the ETSAK motif are critical in positioning the directly interacting residues.
FIGURE 7.
Schematic diagram of the proposed intrinsic and GAP-catalyzed GTP hydrolysis reactions starting with a protonated γ-phosphate. Wat-175 is the nucleophilic water molecule. A, transition state of the intrinsic hydrolysis reaction would be stabilized by sharing of the γ-phosphate proton with a water molecule that donates H-bonds to both Tyr-32 and Gln-61, placing a partial positive charge over the β,γ-bridging oxygen atom. B, transition state for the GAP-catalyzed reaction would be stabilized by the favored interaction between the GAP arginine finger and the β,γ-bridging oxygen of GTP. In this case the interaction between the Arg-789 and the γ-phosphate would be weaker than in the deprotonated case. This is indicated by a gray dashed line. The gray dashed line between the Gln-61 side chain and the nucleophilic water molecule indicates weakening of the interaction in the proposed transition state. A gray solid line indicates stretching of the P–O bond in the transition state in both panels. Both the intrinsic and GAP-catalyzed GTP hydrolysis mechanisms proposed here would stabilize negative charges that accumulate on the β,γ-bridging oxygen atom in a dissociative-like transition state.
Similar articles
- Ras-catalyzed hydrolysis of GTP: a new perspective from model studies.
Maegley KA, Admiraal SJ, Herschlag D. Maegley KA, et al. Proc Natl Acad Sci U S A. 1996 Aug 6;93(16):8160-6. doi: 10.1073/pnas.93.16.8160. Proc Natl Acad Sci U S A. 1996. PMID: 8710841 Free PMC article. Review. - Structural insight into the rearrangement of the switch I region in GTP-bound G12A K-Ras.
Xu S, Long BN, Boris GH, Chen A, Ni S, Kennedy MA. Xu S, et al. Acta Crystallogr D Struct Biol. 2017 Dec 1;73(Pt 12):970-984. doi: 10.1107/S2059798317015418. Epub 2017 Nov 10. Acta Crystallogr D Struct Biol. 2017. PMID: 29199977 - X-ray crystal structures of transforming p21 ras mutants suggest a transition-state stabilization mechanism for GTP hydrolysis.
Privé GG, Milburn MV, Tong L, de Vos AM, Yamaizumi Z, Nishimura S, Kim SH. Privé GG, et al. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3649-53. doi: 10.1073/pnas.89.8.3649. Proc Natl Acad Sci U S A. 1992. PMID: 1565661 Free PMC article. - Conformational states of human rat sarcoma (Ras) protein complexed with its natural ligand GTP and their role for effector interaction and GTP hydrolysis.
Spoerner M, Hozsa C, Poetzl JA, Reiss K, Ganser P, Geyer M, Kalbitzer HR. Spoerner M, et al. J Biol Chem. 2010 Dec 17;285(51):39768-78. doi: 10.1074/jbc.M110.145235. Epub 2010 Oct 11. J Biol Chem. 2010. PMID: 20937837 Free PMC article. - GTP hydrolysis mechanism of Ras-like GTPases.
Li G, Zhang XC. Li G, et al. J Mol Biol. 2004 Jul 23;340(5):921-32. doi: 10.1016/j.jmb.2004.06.007. J Mol Biol. 2004. PMID: 15236956 Review.
Cited by
- Far-reaching effects of tyrosine64 phosphorylation on Ras revealed with BeF3- complexes.
Baumann P, Jin Y. Baumann P, et al. Commun Chem. 2024 Jan 31;7(1):19. doi: 10.1038/s42004-024-01105-6. Commun Chem. 2024. PMID: 38297137 Free PMC article. - Elucidation of Single Hydrogen Bonds in GTPases via Experimental and Theoretical Infrared Spectroscopy.
Mann D, Höweler U, Kötting C, Gerwert K. Mann D, et al. Biophys J. 2017 Jan 10;112(1):66-77. doi: 10.1016/j.bpj.2016.11.3195. Biophys J. 2017. PMID: 28076817 Free PMC article. - Crystal Structure Reveals the Full Ras-Raf Interface and Advances Mechanistic Understanding of Raf Activation.
Cookis T, Mattos C. Cookis T, et al. Biomolecules. 2021 Jul 7;11(7):996. doi: 10.3390/biom11070996. Biomolecules. 2021. PMID: 34356620 Free PMC article. - The Ras Superfamily of Small GTPases in Non-neoplastic Cerebral Diseases.
Qu L, Pan C, He SM, Lang B, Gao GD, Wang XL, Wang Y. Qu L, et al. Front Mol Neurosci. 2019 May 21;12:121. doi: 10.3389/fnmol.2019.00121. eCollection 2019. Front Mol Neurosci. 2019. PMID: 31213978 Free PMC article. Review. - Invited review: Small GTPases and their GAPs.
Mishra AK, Lambright DG. Mishra AK, et al. Biopolymers. 2016 Aug;105(8):431-48. doi: 10.1002/bip.22833. Biopolymers. 2016. PMID: 26972107 Free PMC article. Review.
References
- Wittinghofer A., and Vetter I. R. (2011) Structure-function relationships of the G domain, a canonical switch motif. Annu. Rev. Biochem. 80, 943–971 - PubMed
- Bourne H. R., Sanders D. A., and McCormick F. (1990) The GTPase superfamily: a conserved switch for diverse cell functions. Nature 348, 125–132 - PubMed
- Milburn M. V., Tong L., deVos A. M., Brünger A., Yamaizumi Z., Nishimura S., and Kim S. H. (1990) Molecular switch for signal transduction: differences between active and inactive forms of protooncogenic Ras proteins. Science 247, 939–945 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous