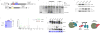Analysis of the Histone H3.1 Interactome: A Suitable Chaperone for the Right Event - PubMed (original) (raw)
Analysis of the Histone H3.1 Interactome: A Suitable Chaperone for the Right Event
Eric I Campos et al. Mol Cell. 2015.
Abstract
Despite minimal disparity at the sequence level, mammalian H3 variants bind to distinct sets of polypeptides. Although histone H3.1 predominates in cycling cells, our knowledge of the soluble complexes that it forms en route to deposition or following eviction from chromatin remains limited. Here, we provide a comprehensive analysis of the H3.1-binding proteome, with emphasis on its interactions with histone chaperones and components of the replication fork. Quantitative mass spectrometry revealed 170 protein interactions, whereas a large-scale biochemical fractionation of H3.1 and associated enzymatic activities uncovered over twenty stable protein complexes in dividing human cells. The sNASP and ASF1 chaperones play pivotal roles in the processing of soluble histones but do not associate with the active CDC45/MCM2-7/GINS (CMG) replicative helicase. We also find TONSL-MMS22L to function as a H3-H4 histone chaperone. It associates with the regulatory MCM5 subunit of the replicative helicase.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
Figure 1
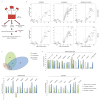
Quantitative mass spectrometry (MS) analyses of H3.1-interacting proteins. (A) Purification Scheme. (B) Quantitative MS analysis of affinity-purified eH3.1 isolated from asynchronous or synchronized, replicating cells. Each volcano plot represents three independent eH3.1 (FLAG) pull-downs plotted against matching mock purifications. The x axis denotes the eH3.1 over mock ratio of MS intensity whereas a false discovery rate (FDR) adapted t-test is plotted on the y axis. (C) Distribution of H3.1-interacting proteins across subcellular compartments. (D) Relative enrichment of histone chaperones, components of the replication fork, and proteins with enzymatic activity within the eH3.1 immunoprecipitates. Data is presented as mean Label-free Quantification (LFQ) ratio +/− SD.
Figure 2
Biochemical isolation of soluble eH3.1 protein complexes. (A) Anion exchange chromatography of eH3.1 affinity purified from either nuclear extracts (left panel) or solubilized chromatin (right panel). Silver stained SDS-PAGE and western analyses revealed co-eluting proteins (top panels), whereas the extracts were further essayed for intrinsic enzymatic activities towards histones using radiolabeled acetyl-CoA, SAM and ATP (bottom panels). The elution points above the gels denote fractions where eH3.1 protein levels peaked. These fractions, as well as fractions containing enzymatic activities towards histones were further fractionated (see Figure S2, Figure S3). (B) H3.1 interactome based on the biochemical purification of soluble eH3.1 protein complexes. Proteins validated in the quantitative MS analysis are highlighted in yellow. Solid grey and dashed red lines respectively represent interactions reported in STRING, and interactions found through the biochemical purification of soluble eH3.1. NC: negative control, PC: positive control, KAT: lysine acetyltransferase, KMT: lysine methyltransferase, CBB: Coomassie Brilliant Blue.
Figure 3
Nucleosome disassembly. (A) A radiolabeled PCNA probe is loaded onto a linear DNA template, flanked by a single nucleosome at one extremity and a biotin-streptavidin block at the other. DNA flows into the exclusion volume when applied onto a gel filtration spin column, along with the radiolabeled PCNA trapped by the nucleosome. The radioactive probe is free to slide on DNA but falls off and remains in the inclusion volume if histones are removed. The assay quantifies the amount of radioactive PCNA in the exclusion volume by Cherenkov counting. (B) Testing nucleosome disassembly activity within affinity-purified eH3.1 from nuclear fractions (Figure 2). Data represents mean [32P]-PCNA retention +/− SD. (C) Mass spectrometry analysis (peptide counts) of the three fractions exhibiting histone eviction.
Figure 4
Molecular functions of nuclear sNASP and ASF1B. (A) Nuclear sNASP co-elutes with HAT1 and evicted histones. (B) Both sNASP-HAT1 complexes (with or without evicted histones) are enzymatically active. Left panel: Western analysis of peak eH3.1 fractions containing sNASP with evicted or new histones (precipitated at 3.4 and 2.8 M ammonium sulfate, respectively). Right panel: Acetyltransferase activity towards histones demonstrated by autoradiography of acetyl-[3H] incorporation. (C) Nuclear, but not cytosolic sNASP co-precipitates histones with marks characteristic of eu- and hetero-chromatin. (D) In vitro PRC2 methyltransferase assay. sNASP-bound histones are poor substrates for the PRC2 complex compared to free soluble (H3-H4)2 tetramers. (E) In vivo crosslinking of replicating 293 cells (left panel), and mass spectrometry analysis of crosslinked, immunoprecipitated, ASF1B.
Figure 5
TONSL is a histone chaperone that binds H3K9me1. (A) Primary structures of human TONSL and the similar yeast protein, Dia2. (B) Supercoiling assay demonstrating the histone chaperone ability of TONSL. (C) Pull-down assay utilizing immobilized histone peptides testing binding preference by recombinant TONSL and HP1. (D) MS/MS HCD spectrum of the (M + H)+4 ion of cross-linked peptides between TONSL (TRP repeat) and the C-terminus of MCM5. N-terminal fragment ions (b) are indicated in blue and C-terminal fragment ions (y) are indicated in green and red. The mass accuracy for precursor ion is better than 1 ppm and mass accuracy of all the fragment ions is better than 10 ppm. (E) Immobilized TONSL binds to in vitro translated MCM5. (F) Model for TONSL-MMS22L at the replication fork: Upon recruitment to stalled replication forks TONSL-MMS22L may maintain the CMG helicase inactive by binding to MCM5.
Similar articles
- Distinct histone H3-H4 binding modes of sNASP reveal the basis for cooperation and competition of histone chaperones.
Liu CP, Jin W, Hu J, Wang M, Chen J, Li G, Xu RM. Liu CP, et al. Genes Dev. 2021 Dec 1;35(23-24):1610-1624. doi: 10.1101/gad.349100.121. Epub 2021 Nov 24. Genes Dev. 2021. PMID: 34819355 Free PMC article. - Structural insight into how the human helicase subunit MCM2 may act as a histone chaperone together with ASF1 at the replication fork.
Richet N, Liu D, Legrand P, Velours C, Corpet A, Gaubert A, Bakail M, Moal-Raisin G, Guerois R, Compper C, Besle A, Guichard B, Almouzni G, Ochsenbein F. Richet N, et al. Nucleic Acids Res. 2015 Feb 18;43(3):1905-17. doi: 10.1093/nar/gkv021. Epub 2015 Jan 23. Nucleic Acids Res. 2015. PMID: 25618846 Free PMC article. - Roles of histone chaperone CIA/Asf1 in nascent DNA elongation during nucleosome replication.
Ishikawa K, Ohsumi T, Tada S, Natsume R, Kundu LR, Nozaki N, Senda T, Enomoto T, Horikoshi M, Seki M. Ishikawa K, et al. Genes Cells. 2011 Oct;16(10):1050-62. doi: 10.1111/j.1365-2443.2011.01549.x. Epub 2011 Sep 7. Genes Cells. 2011. PMID: 21895891 - A Molecular Prospective for HIRA Complex Assembly and H3.3-Specific Histone Chaperone Function.
Ricketts MD, Marmorstein R. Ricketts MD, et al. J Mol Biol. 2017 Jun 30;429(13):1924-1933. doi: 10.1016/j.jmb.2016.11.010. Epub 2016 Nov 19. J Mol Biol. 2017. PMID: 27871933 Free PMC article. Review. - Histone transfer among chaperones.
Liu WH, Churchill ME. Liu WH, et al. Biochem Soc Trans. 2012 Apr;40(2):357-63. doi: 10.1042/BST20110737. Biochem Soc Trans. 2012. PMID: 22435812 Free PMC article. Review.
Cited by
- A specific role for importin-5 and NASP in the import and nuclear hand-off of monomeric H3.
Pardal AJ, Bowman AJ. Pardal AJ, et al. Elife. 2022 Sep 6;11:e81755. doi: 10.7554/eLife.81755. Elife. 2022. PMID: 36066346 Free PMC article. - p53 ensures the normal behavior and modification of G1/S-specific histone H3.1 in the nucleus.
Oikawa T, Hasegawa J, Handa H, Ohnishi N, Onodera Y, Hashimoto A, Sasaki J, Sasaki T, Ueda K, Sabe H. Oikawa T, et al. Life Sci Alliance. 2024 Jun 21;7(9):e202402835. doi: 10.26508/lsa.202402835. Print 2024 Sep. Life Sci Alliance. 2024. PMID: 38906678 Free PMC article. - NASP maintains histone H3-H4 homeostasis through two distinct H3 binding modes.
Bao H, Carraro M, Flury V, Liu Y, Luo M, Chen L, Groth A, Huang H. Bao H, et al. Nucleic Acids Res. 2022 May 20;50(9):5349-5368. doi: 10.1093/nar/gkac303. Nucleic Acids Res. 2022. PMID: 35489058 Free PMC article. - Function of cofactor Akirin2 in the regulation of gene expression in model human Caucasian neutrophil-like HL60 cells.
Artigas-Jerónimo S, Villar M, Estrada-Peña A, Velázquez-Campoy A, Alberdi P, de la Fuente J. Artigas-Jerónimo S, et al. Biosci Rep. 2021 Jul 30;41(7):BSR20211120. doi: 10.1042/BSR20211120. Biosci Rep. 2021. PMID: 34291801 Free PMC article. - DNAJC9 integrates heat shock molecular chaperones into the histone chaperone network.
Hammond CM, Bao H, Hendriks IA, Carraro M, García-Nieto A, Liu Y, Reverón-Gómez N, Spanos C, Chen L, Rappsilber J, Nielsen ML, Patel DJ, Huang H, Groth A. Hammond CM, et al. Mol Cell. 2021 Jun 17;81(12):2533-2548.e9. doi: 10.1016/j.molcel.2021.03.041. Epub 2021 Apr 14. Mol Cell. 2021. PMID: 33857403 Free PMC article.
References
- Annunziato AT. Assembling chromatin: The long and winding road. Biochim Biophys Acta. 2012;1819:196–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA199652/CA/NCI NIH HHS/United States
- P30 CA016087/CA/NCI NIH HHS/United States
- GM-64844/GM/NIGMS NIH HHS/United States
- R37-37120/PHS HHS/United States
- R01 GM037120/GM/NIGMS NIH HHS/United States
- R01 GM064844/GM/NIGMS NIH HHS/United States
- S10 OD016400/OD/NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous