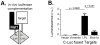Mst1 Kinase Regulates the Actin-Bundling Protein L-Plastin To Promote T Cell Migration - PubMed (original) (raw)
Mst1 Kinase Regulates the Actin-Bundling Protein L-Plastin To Promote T Cell Migration
Xiaolu Xu et al. J Immunol. 2016.
Abstract
Exploring the mechanisms controlling lymphocyte trafficking is essential for understanding the function of the immune system and the pathophysiology of immunodeficiencies. The mammalian Ste20-like kinase 1 (Mst1) has been identified as a critical signaling mediator of T cell migration, and loss of Mst1 results in immunodeficiency disease. Although Mst1 is known to support T cell migration through induction of cell polarization and lamellipodial formation, the downstream effectors of Mst1 are incompletely defined. Mice deficient for the actin-bundling protein L-plastin (LPL) have phenotypes similar to mice lacking Mst1, including decreased T cell polarization, lamellipodial formation, and cell migration. We therefore asked whether LPL functions downstream of Mst1. The regulatory N-terminal domain of LPL contains a consensus Mst1 phosphorylation site at Thr(89) We found that Mst1 can phosphorylate LPL in vitro and that Mst1 can interact with LPL in cells. Removal of the Mst1 phosphorylation site by mutating Thr(89) to Ala impaired localization of LPL to the actin-rich lamellipodia of T cells. Expression of the T89A LPL mutant failed to restore migration of LPL-deficient T cells in vitro. Furthermore, expression of T89A LPL in LPL-deficient hematopoietic cells, using bone marrow chimeras, failed to rescue the phenotype of decreased thymic egress. These results identify LPL as a key effector of Mst1 and establish a novel mechanism linking a signaling intermediate to an actin-binding protein critical to T cell migration.
Copyright © 2016 by The American Association of Immunologists, Inc.
Figures
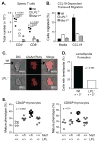
Figure 1. The phenotypes of Mst1h/h and LPL−/− mice are similar
(A) Total numbers of CD4+ and CD8+ T cells in spleens of Wt, LPL−/−, and Mst1h/h mice. (B) Migration efficiency of CD4+ T cells from Wt, LPL−/−, and Mst1h/h mice. Cells were incubated in the upper well of 5-μm Transwell membrane chambers; the bottom well contained 100 ng/ml CCL19. Data are representative of at least two independent experiments. (C) Impaired lamellipodial formation in T cells from LPL−/− mice. Arrow indicates flat lamellipodium at leading edge of Wt cell. Representative images. (D) Quantification of percentages of T cells from WT and LPL−/− mice that generate lamellipodia, from three independent experiments. P-value determined using Fisher’s exact test. (E) Increased proportions of CD4SP and CD8SP thymocytes from Mst1+/h LPL+/− mice exhibit a mature (CD69negCD24neg) phenotype, indicating reduced thymic egress. Each symbol represents data from one animal; data from age- and sex-matched mice displayed; line at median, p-value determined with one-way ANOVA.
Figure 2. Mst1 and LPL interact in 293T cells
(A) Schematic representation of method to test Mst1-LPL interaction using luciferase reconstitution assay. (B) Interactions between Mst1 and target proteins were evaluated by their ability to reconstitute luciferase activity. 293T cells were co-transfected with Mst1 fused to the N-terminal domain of luciferase (N-Luc) and the indicated targets fused to the C-terminal domain of luciferase (C-Luc). Empty C-Luc and Vimentin-C-Luc fusion proteins were used as negative controls. C-Luc fused to Mob1a, a known Mst1 target, was used as a positive control. Data are representative of 2 – 3 independent experiments; p-value determined using Mann-Whitney.
Figure 3. Mst1 can phosphorylate the Thr89 residue in the N-terminal portion of LPL
(A) Schematic of LPL regulatory and actin-binding domains. The N-terminal regulatory domain contains a known phosphorylation site at Ser5 (S5; star), two EF-hands that generate a calcium binding site (EF), a calmodulin binding site (Cal), and a putative Mst1-phosphorylation site centered on Thr89 (T89). The C-terminal domain contains four calponin homology (CH) domains, each pair of which creates an actin-binding domain. (B) Alignment of the portion of the regulatory domain of all three mouse plastins that contain the Mst1 phosphorylation site, by ClustalW. “*” indicates identity, “:” indicates similarity. (C) Immunoblot of LPL in whole cell lysates of CD4+ T cells isolated from Wt and Mst1h/h mice, separated by SDS-PAGE. (D) Upper panel: In vitro phosphorylation of Wt or T89A mutant LPL regulatory domain (residues 1-112) by Wt or kinase-defective (kd) Mst1 with 32P, visualized by autoradiography. Lower panel: Recombinant LPL input proteins were visualized by Coomassie Blue staining. (E) Mst1-mediated phosphorylation of full length Wt or T89A mutant LPL visualized by immunoblot analysis of transfected 293T cell lysates with an antibody specific for phosphoThr-X-Arg.
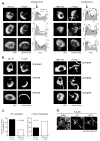
Figure 4. Phosphorylation of Thr89 supports LPL localization to the lamellipod
(A, B) Purified CD4+ T cells from LPL−/− mice were reconstituted with Wt or T89A LPL-GFP and with LifeAct-Ruby, stimulated with CCL19 in ICAM-1 coated chamberslides, fixed, and visualized by confocal microscopy. (A) Three representative cells are shown for Wt LPL-GFP and for T89A LPL-GFP. Intensity profiles of LifeAct-Ruby (filled, gray) and GFP (Wt LPL or T89A LPL; solid line) were calculated along the white lines indicated on the images. On the plots, the X-axis is pixel numbers; the left Y-axis is LifeAct-Ruby intensity (relative units); and the right Y-axis indicates GFP intensity (relative units). An example of “clumping” is indicated by the white arrow. (B) Three representative cells are shown for Wt LPL-GFP and for T89A LPL-GFP. Arrows indicate “clumped” morphology of F-actin. (C) Confocal micrographs of cells expressing Wt or T89A LPL-GFP from (A, B) were blindly scored for GFP enrichment in the lamellipodia and for F-actin clumping. Data combined from three independent experiments; p-value determined by Fisher’s exact test. (D) AlexaFluor488-conjugated rabbit muscle actin was polymerized prior to addition of kinase buffer only (buffer) or recombinant LPL previously incubated with Mst1 (pLPL) or without Mst1 (LPL). Actin filaments and bundles were visualized by TIRF microscopy.
Figure 5. Thr89 phosphorylation is not required for interaction with CaM
Cell extracts containing GFP, Wt LPL-GFP or T89A LPL-GFP fusion proteins were incubated with CaM-coated beads in the presence of EGTA or 1 μM CaCl2. LPL binding to CaM was assessed by immunoblots with anti-GFP and anti-CaM. Data are from one experiment, representative of four independent experiments.
Figure 6. T89A LPL does not fully rescue T cell migration in vitro or in vivo
(A) CD4+ T cells purified from LPL−/− mice were reconstituted with Wt or T89A LPL-GFP. The mixed population containing reconstituted (GFP**+, Wt or T89A LPL) and non-reconstituted (GFPneg**, LPL-deficient) cells were seeded into the top well of a 5-μm transwell with 100 ng/ml CCL19 in the bottom well. Cells were counted by flow cytometry. Activated CD4+ T cells from Wt mice are included as a positive control. Data are normalized to migratory efficiency of GFPneg (non-reconstituted, LPL-deficient) T cells and are representative of three independent experiments; p-value determined by two-way ANOVA. (B) Schematic representation of generation of bone marrow chimeras. HSCs from CD45.2+ LPL−/− donors were infected with lentiviral constructs encoding Wt or T89A LPL-GFP in LPL−/− HSCs, then transferred into irradiated CD45.1+ Wt recipients. Thymocytes were analyzed 8 wk after engraftment. (C) Thymic phenotype of bone marrow chimeric mice re-expressing Wt LPL or T89A LPL in an LPL−/− background. Non-reconstituted LPL−/− cells were identified as CD45.2+-GFPneg and Wt or T89A LPL-reconstituted cells were identified as CD45.2+-GFP+. Data are from one experiment, representative of two independent experiments.
Similar articles
- Efficient T Cell Migration and Activation Require L-Plastin.
Joshi H, Morley SC. Joshi H, et al. Front Immunol. 2022 Jun 29;13:916137. doi: 10.3389/fimmu.2022.916137. eCollection 2022. Front Immunol. 2022. PMID: 35844504 Free PMC article. Review. - Actin-bundling protein L-plastin regulates T cell activation.
Wang C, Morley SC, Donermeyer D, Peng I, Lee WP, Devoss J, Danilenko DM, Lin Z, Zhang J, Zhou J, Allen PM, Brown EJ. Wang C, et al. J Immunol. 2010 Dec 15;185(12):7487-97. doi: 10.4049/jimmunol.1001424. Epub 2010 Nov 12. J Immunol. 2010. PMID: 21076065 Free PMC article. - The actin-bundling protein L-plastin dissociates CCR7 proximal signaling from CCR7-induced motility.
Morley SC, Wang C, Lo WL, Lio CW, Zinselmeyer BH, Miller MJ, Brown EJ, Allen PM. Morley SC, et al. J Immunol. 2010 Apr 1;184(7):3628-38. doi: 10.4049/jimmunol.0903851. Epub 2010 Mar 1. J Immunol. 2010. PMID: 20194718 Free PMC article. - L-plastin regulates polarization and migration in chemokine-stimulated human T lymphocytes.
Freeley M, O'Dowd F, Paul T, Kashanin D, Davies A, Kelleher D, Long A. Freeley M, et al. J Immunol. 2012 Jun 15;188(12):6357-70. doi: 10.4049/jimmunol.1103242. Epub 2012 May 11. J Immunol. 2012. PMID: 22581862 - The actin-bundling protein L-plastin supports T-cell motility and activation.
Morley SC. Morley SC. Immunol Rev. 2013 Nov;256(1):48-62. doi: 10.1111/imr.12102. Immunol Rev. 2013. PMID: 24117812 Free PMC article. Review.
Cited by
- Efficient T Cell Migration and Activation Require L-Plastin.
Joshi H, Morley SC. Joshi H, et al. Front Immunol. 2022 Jun 29;13:916137. doi: 10.3389/fimmu.2022.916137. eCollection 2022. Front Immunol. 2022. PMID: 35844504 Free PMC article. Review. - The Role of Mst1 in Lymphocyte Homeostasis and Function.
Cheng J, Jing Y, Kang D, Yang L, Li J, Yu Z, Peng Z, Li X, Wei Y, Gong Q, Miron RJ, Zhang Y, Liu C. Cheng J, et al. Front Immunol. 2018 Feb 5;9:149. doi: 10.3389/fimmu.2018.00149. eCollection 2018. Front Immunol. 2018. PMID: 29459865 Free PMC article. Review. - WASP and Mst1 coregulate B-cell development and B-cell receptor signaling.
Huang L, Sun X, Yang D, Dai X, Jiang P, Bai X, Zhang Y, Wang J, Li W, Miller H, Song W, Treanor B, Zhao X, Liu C. Huang L, et al. Blood Adv. 2020 Feb 11;4(3):573-585. doi: 10.1182/bloodadvances.2018027870. Blood Adv. 2020. PMID: 32045478 Free PMC article. - L-Plastin Phosphorylation: Possible Regulation by a TNFR1 Signaling Cascade in Osteoclasts.
Chellaiah MA. Chellaiah MA. Cells. 2021 Sep 15;10(9):2432. doi: 10.3390/cells10092432. Cells. 2021. PMID: 34572081 Free PMC article. - Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine.
Dey A, Varelas X, Guan KL. Dey A, et al. Nat Rev Drug Discov. 2020 Jul;19(7):480-494. doi: 10.1038/s41573-020-0070-z. Epub 2020 Jun 17. Nat Rev Drug Discov. 2020. PMID: 32555376 Free PMC article. Review.
References
- Cyster JG, Schwab SR. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol. 2012;30:69–94. - PubMed
- Burkhardt JK, Carrizosa E, Shaffer MH. The actin cytoskeleton in T cell activation. Annu Rev Immunol. 2008;26:233–259. - PubMed
- Krummel MF, Macara I. Maintenance and modulation of T cell polarity. Nat Immunol. 2006;7:1143–1149. - PubMed
- Nehme NT, Pachlopnik Schmid J, Debeurme F, Andre-Schmutz I, Lim A, Nitschke P, Rieux-Laucat F, Lutz P, Picard C, Mahlaoui N, Fischer A, de Saint Basile G. MST1 mutations in autosomal recessive primary immunodeficiency characterized by defective naive T-cell survival. Blood. 2012;119:3458–3468. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- R01 GM038542/GM/NIGMS NIH HHS/United States
- P30 CA023108/CA/NCI NIH HHS/United States
- S10 RR024688/RR/NCRR NIH HHS/United States
- R01 AI104732/AI/NIAID NIH HHS/United States
- R35 GM118171/GM/NIGMS NIH HHS/United States
- R01 AI089805/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous