Actin bundling by dynamin 2 and cortactin is implicated in cell migration by stabilizing filopodia in human non-small cell lung carcinoma cells - PubMed (original) (raw)
Actin bundling by dynamin 2 and cortactin is implicated in cell migration by stabilizing filopodia in human non-small cell lung carcinoma cells
Hiroshi Yamada et al. Int J Oncol. 2016 Sep.
Abstract
The endocytic protein dynamin participates in the formation of actin-based membrane protrusions such as podosomes, pseudopodia, and invadopodia, which facilitate cancer cell migration, invasion, and metastasis. However, the role of dynamin in the formation of actin-based membrane protrusions at the leading edge of cancer cells is unclear. In this study, we demonstrate that the ubiquitously expressed dynamin 2 isoform facilitates cell migration by stabilizing F-actin bundles in filopodia of the lung cancer cell line H1299. Pharmacological inhibition of dynamin 2 decreased cell migration and filopodial formation. Furthermore, dynamin 2 and cortactin mostly colocalized along F-actin bundles in filopodia of serum-stimulated H1299 cells by immunofluorescent and immunoelectron microscopy. Knockdown of dynamin 2 or cortactin inhibited the formation of filopodia in serum-stimulated H1299 cells, concomitant with a loss of F-actin bundles. Expression of wild-type cortactin rescued the punctate-like localization of dynamin 2 and filopodial formation. The incubation of dynamin 2 and cortactin with F-actin induced the formation of long and thick actin bundles, with these proteins colocalizing at F-actin bundles. A depolymerization assay revealed that dynamin 2 and cortactin increased the stability of F-actin bundles. These results indicate that dynamin 2 and cortactin participate in cell migration by stabilizing F-actin bundles in filopodia. Taken together, these findings suggest that dynamin might be a possible molecular target for anticancer therapy.
Figures
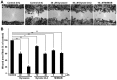
Figure 1
Dynamin GTPase inhibitors inhibit the migration of H1299 cells. (A) Representative images acquired by light microscopy showing cell migration in a wound healing assay. Confluent H1299 cells were wounded and then incubated for 8 h in the presence or absence of dynamin GTPase inhibitors at the indicated concentrations. For the negative control (control 0 h), cells were incubated with 1% DMSO. Scale bar, 200 μm. (B) Morphometric analysis of the wound area filled by migrating cells after treatment with inhibitors at the indicated concentrations. The changes were normalized to the control. Results represent the means ± SEM of three independent experiments.
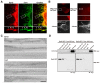
Figure 2
Dynamin 2 colocalizes with cortactin along F-actin bundles in filopodia of serum-stimulated H1299 cells. (A) Colocalization of dynamin 2 (Dyn2, left) and cortactin (Cort, middle) by double-immunofluorescent staining in filopodia of serum-stimulated H1299 cells. Boxed areas correspond to enlarged images shown below. Dynamin 2- and cortactin-positive puncta were present periodically along F-actin bundles in filopodia (arrowheads). Scale bar, 5 μm (upper panels), 1.6 μm (lower panels). (B) In the negative controls, the primary antibodies were omitted for dynamin 2 (left) and cortactin (right). Boxed areas correspond to enlarged images shown below. Bar, 10 μm (top and bottom panels), 2.8 μm (middle panels). (C) Representative images acquired by immunoelectron microscopy showing the localization of dynamin 2 (top three panels) and cortactin (bottom three panels) in filopodia of serum-stimulated H1299 cells. Immunoreactive dynamin 2 and cortactin were present along F-actin bundles (arrowheads). Scale bar, 20 nm. (D) Immunoprecipitation (IP) results demonstrating an in vivo interaction between dynamin 2 and cortactin. H1299 cells were co-transfected with GFP-tagged dynamin 2 (Dyn2-GFP) and either myc-tagged wild-type cortactin (Cort WT-myc, left) or cortactin ΔSH3 (Cort ΔSH3-myc, right). The protein complexes were immunoprecipitated using a polyclonal anti-myc antibody or preimmune IgG (IgG), and then visualized by western blotting (WB) with monoclonal anti-GFP or anti-myc antibodies. Total cell lysates (4, 10 and 20 μg) were also analyzed (input).
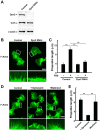
Figure 3
Knockdown of dynamin 2 decreases filopodial formation in H1299 cells. (A) Western blotting showing knockdown of dynamin 2 (Dyn2) expression by RNAi in H1299 cells. β-actin served as the control. Three micrograms of cell lysate from each sample was analyzed by gel electrophoresis. (B) F-actin was visualized in H1299 cells by Alexa Fluor 488-phalloidin staining after knockdown of dynamin 2. Extensive filopodial formation was observed in cells after serum stimulation (left). Filopodial formation was inhibited in dynamin 2-silenced cells (right). Boxed areas correspond to enlarged images shown below. Scale bar, 20 μm (upper panels), 5 μm (lower panels). (C) Filopodial length in H1299 cells cultured in the presence or absence of serum. The cells were visualized by fluorescent confocal microscopy, and filopodial length was measured as described in Materials and methods. (D) Inhibition of filopodial formation by dynasore in serum-stimulated H1299 cells. Serum-starved cells were incubated with 240 μM dynasore for 30 min, and then stimulated with 10% FBS for 45 min in the presence of 240 μM dynasore (middle). Thereafter, dynasore was removed, and the cells were incubated in serum-containing medium for 45 min (right). For the negative control, cells were cultured in the presence of 3% DMSO (left). All steps were performed at 37°C. (E) Analysis of filopodial formation in the H1299 cells shown in (D). The cells were analyzed by fluorescent confocal microscopy, and filopodial length was measured. Results in (C) and (E) represent the means ± SEM from three independent experiments.
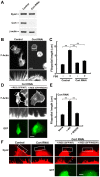
Figure 4
Knockdown of cortactin decreases filopodial formation in H1299 cells. (A) Western blotting showing knockdown of cortactin expression by RNAi in H1299 cells. β-actin was used as the control. Three micrograms of cell lysate from each sample was analyzed by gel electrophoresis. (B) F-actin was visualized in serum-stimulated H1299 cells by Alexa Fluor 488-phalloidin staining. Boxed areas correspond to enlarged images shown below. Similar to results from dynamin 2-depleted cells, filopodial formation decreased in cortactin-depleted cells (right). Scale bar, 20 μm (upper panels), 5 μm (lower panels). (C) Analysis of filopodial formation in H1299 cells cultured with or without serum. The samples were analyzed by fluorescent confocal microscopy, and the filopodial length was measured. (D) Expression of wild-type cortactin rescues filopodial formation. Cortactin-depleted H1299 cells were transfected with rat wild-type cortactin (left) or cortactin W525K (right) cloned into the pIRES2-AcGFP1 expression vector. Boxed areas correspond to enlarged images shown (middle panels). Transfected cells were identified by GFP expression (bottom panels). Scale bar, 20 μm (top and bottom panels), 3.5 μm (middle panels). (E) Analysis of filopodial formation in H1299 cells. The samples were analyzed by fluorescent confocal microscopy, and the filopodial length was measured. Results in (C) and (E) represent the means ± SEM from three independent experiments. (F) Rescue of the punctate-like localization of dynamin 2 along F-actin bundles in filopodia by re-expression of cortactin in cortactin-depleted cells. Cortactin-depleted H1299 cells (left panels) were transfected with rat wild-type cortactin or cortactin W525K cloned into the pIRES2-AcGFP1 expression vector (right panels). The cells were immunostained with an anti-dynamin 2 antibodies. Boxed areas correspond to enlarged images shown below. Transfected cells were identified by GFP expression (right bottom panels). Scale bar, 5 μm (top and right bottom panels), 2.3 μm (left bottom and right middle panels).
Figure 5
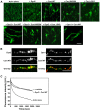
Actin bundling by dynamin 2 and cortactin stabilizes F-actin bundles. (A) Long F-actin bundles were formed in the presence of dynamin 2 and cortactin (lower left). Preformed F-actin (3.3 μM) was incubated with or without the indicated proteins (5 μM each). F-actin was visualized with Alexa Fluor 488-phalloidin. Scale bar, 30 μm. (B) Representative images acquired by fluorescent microscopy showing the localization of dynamin and cortactin along F-actin bundles. Actin bundles were formed in vitro by incubating dynamin 2 with wild-type cortactin (left) or dynamin 1 and wild-type cortactin (right). Protein colocalization was performed by double-immunofluorescence. Scale bar, 2 μm. (C) Kinetics of F-actin disassembly induced in 10-fold diluted preformed pyrene-labeled F-actin solution with buffer. F-actin bundles disassembled in the presence of dynamin 2 and cortactin, as well as in the presence of 5 μM α-actinin. The rate of F-actin bundle disassembly was measured by pyrene-fluorescence.
Figure 6
Putative role of the dynamin 2-cortactin complex in cancer cell migration. The dynamin 2-cortactin complex bundles actin filaments, which stabilize filopodia. In addition, the dynamin 2-cortactin complex participates in the formation of pseudopodia. The regulation of actin by dynamin 2 and cortactin may also be involved in cancer cell invasion and metastasis.
Similar articles
- Stabilization of actin bundles by a dynamin 1/cortactin ring complex is necessary for growth cone filopodia.
Yamada H, Abe T, Satoh A, Okazaki N, Tago S, Kobayashi K, Yoshida Y, Oda Y, Watanabe M, Tomizawa K, Matsui H, Takei K. Yamada H, et al. J Neurosci. 2013 Mar 6;33(10):4514-26. doi: 10.1523/JNEUROSCI.2762-12.2013. J Neurosci. 2013. PMID: 23467367 Free PMC article. - Phosphorylation of cortactin by cyclin-dependent kinase 5 modulates actin bundling by the dynamin 1-cortactin ring-like complex and formation of filopodia and lamellipodia in NG108-15 glioma-derived cells.
Abe T, La TM, Miyagaki Y, Oya E, Wei FY, Sumida K, Fujise K, Takeda T, Tomizawa K, Takei K, Yamada H. Abe T, et al. Int J Oncol. 2019 Feb;54(2):550-558. doi: 10.3892/ijo.2018.4663. Epub 2018 Dec 11. Int J Oncol. 2019. PMID: 30570111 Free PMC article. - Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape.
McNiven MA, Kim L, Krueger EW, Orth JD, Cao H, Wong TW. McNiven MA, et al. J Cell Biol. 2000 Oct 2;151(1):187-98. doi: 10.1083/jcb.151.1.187. J Cell Biol. 2000. PMID: 11018064 Free PMC article. - Dynamin as a mover and pincher during cell migration and invasion.
Kruchten AE, McNiven MA. Kruchten AE, et al. J Cell Sci. 2006 May 1;119(Pt 9):1683-90. doi: 10.1242/jcs.02963. J Cell Sci. 2006. PMID: 16636070 Review. - Cortactin branches out: roles in regulating protrusive actin dynamics.
Ammer AG, Weed SA. Ammer AG, et al. Cell Motil Cytoskeleton. 2008 Sep;65(9):687-707. doi: 10.1002/cm.20296. Cell Motil Cytoskeleton. 2008. PMID: 18615630 Free PMC article. Review.
Cited by
- The Expression of Dynamin 1, 2, and 3 in Human Hepatocellular Carcinoma and Patient Prognosis.
Tian M, Yang X, Li Y, Guo S. Tian M, et al. Med Sci Monit. 2020 Jun 23;26:e923359. doi: 10.12659/MSM.923359. Med Sci Monit. 2020. PMID: 32573516 Free PMC article. - Role of Endocytosis Proteins in Gefitinib-Mediated EGFR Internalisation in Glioma Cells.
Cruz Da Silva E, Choulier L, Thevenard-Devy J, Schneider C, Carl P, Rondé P, Dedieu S, Lehmann M. Cruz Da Silva E, et al. Cells. 2021 Nov 21;10(11):3258. doi: 10.3390/cells10113258. Cells. 2021. PMID: 34831480 Free PMC article. - Gain-of-Function Dynamin-2 Mutations Linked to Centronuclear Myopathy Impair Ca2+-Induced Exocytosis in Human Myoblasts.
Bayonés L, Guerra-Fernández MJ, Hinostroza F, Báez-Matus X, Vásquez-Navarrete J, Gallo LI, Parra S, Martínez AD, González-Jamett A, Marengo FD, Cárdenas AM. Bayonés L, et al. Int J Mol Sci. 2022 Sep 8;23(18):10363. doi: 10.3390/ijms231810363. Int J Mol Sci. 2022. PMID: 36142275 Free PMC article. - From Transcriptomics, Metabolomics to Functional Studies: Extracellular ATP Induces TGF-β-Like Epithelial Mesenchymal Transition in Lung Cancer Cells.
Evers M, Song J, Shriwas P, Greenbaum HS, Chen X. Evers M, et al. Front Oncol. 2022 Jun 30;12:912065. doi: 10.3389/fonc.2022.912065. eCollection 2022. Front Oncol. 2022. PMID: 35847855 Free PMC article. - DNM1: A Prognostic Biomarker Associated with Immune Infiltration in Colon Cancer-A Study Based on TCGA Database.
Hu M, Gu J, Su W, Zhang Z, Zhu B, Wang Q, Xing C. Hu M, et al. Biomed Res Int. 2021 Nov 30;2021:4896106. doi: 10.1155/2021/4896106. eCollection 2021. Biomed Res Int. 2021. PMID: 34888380 Free PMC article. Retracted.
References
- Takei K, Slepnev VI, Haucke V, De Camilli P. Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat Cell Biol. 1999;1:33–39. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous