Cannabidiol Modulates the Immunophenotype and Inhibits the Activation of the Inflammasome in Human Gingival Mesenchymal Stem Cells - PubMed (original) (raw)
Cannabidiol Modulates the Immunophenotype and Inhibits the Activation of the Inflammasome in Human Gingival Mesenchymal Stem Cells
Rosaliana Libro et al. Front Physiol. 2016.
Abstract
Human Gingival Mesenchymal Stem Cells (hGMSCs) are multipotential cells that can expand and differentiate in culture under specific and standardized conditions. In the present study, we have investigated whether in vitro pre-treatment of hGMSCs with Cannabidiol (CBD) can influence their expression profile, improving the therapeutic potential of this cell culture. Following CBD treatment (5 μM) for 24 h, gene expression analysis through Next Generation Sequencing (NGS) has revealed several genes differentially expressed between CBD-treated hGMSCs (CBD-hGMSCs) and control cells (CTR-hGMSCs) that were linked to inflammation and apoptosis. In particular, we have demonstrated that CBD treatment in hGMSCs prevented the activation of the NALP3-inflammasome pathway by suppressing the levels of NALP3, CASP1, and IL18, and in parallel, inhibited apoptosis, as demonstrated by the suppression of Bax. CBD treatment was also able to modulate the expression of the well-known mesenchymal stem cell markers (CD13, CD29, CD73, CD44, CD90, and CD166), and other surface antigens. Specifically, CBD led to the downregulation of genes codifying for antigens involved in the activation of the immune system (CD109, CD151, CD40, CD46, CD59, CD68, CD81, CD82, CD99), while it led to the upregulation of those implicated in the inhibition of the immune responses (CD47, CD55, CD276). In conclusion, the present study will provide a new simple and reproducible method for preconditioning hGMSCs with CBD, before transplantation, as an interesting strategy for improving the hGMSCs molecular phenotype, reducing the risk of immune or inflammatory reactions in the host, and in parallel, for increasing their survival and thus, their long-term therapeutic efficacy.
Keywords: cannabidiol; human gingival mesenchymal stem cells; immunophenotype; inflammasome; next generation sequencing.
Figures
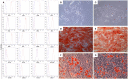
Figure 1
Characterization of gingiva-derived mesenchymal stem cells (hGMSCs). (A) Flow cytometry phenotype of hGMSCs at the second passage during in vitro cultures of surface (CD13, CD14, CD29, CD31, CD34, CD44, CD45, CD73, CD90, CD105, CD117, CD133, CD146, CD166, CD326, HLA-ABC, and HLA-DR) and intracellular (SSEA4, Oct3/4, Sox2, and NANOG) marker expression levels were detected. Red histograms show the distribution of each antigen expression, whereas Blue histograms represent the distribution of the respective background control. (B) The gingival connective tissue-derived mesenchymal stem cells showed colony-forming potency and plastic-adherent characteristics at day 6. (C) CBD-hGMSCs and CTR-hGMSCs showed spindle-shaped, fibroblast-like morphology under light microscope. (D) Osteogenic differentiation at day 21 after Alizarin Red S staining highlighted calcium deposits in CTR-hGMSCs and in (E) CBD-hGMSCs. (F) CTR-hGMSCs and (G) CBD-hGMSCs induced to a dipogenic differentiation after 28 days showed many oil droplets at cytoplasmic level stained with Oil Red O solution. Mag: 10X; bar: 200 μm.
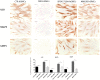
Figure 2
Immunocytochemical staining for IL18, NALP3 and CASP1. CBD-hGMSCs showed a negative staining for IL-18, NALP3, and CASP1 compared to CTR-hGMSCs. Instead, hGMSCs-SR141716A and hGMSCs-AM630 showed a significant positive staining for IL-18, NALP3, and CASP1 compared to CBD-hGMSCs. The graph represented the densytometric quantitative analysis. For IL-18 CTR-hGMSCs vs. CBD-hGMSCs ***p < 0.001; SR141716A-hGMSCs vs. AM630-hGMSCs ****p < 0.0001. For NALP3 CTR-hGMSCs vs. CBD-hGMSCs **p < 0.01; SR141716A-hGMSCs vs. AM630-hGMSCs **p < 0.01. For CASP-1 CTR-hGMSCs vs. CBD-hGMSCs ***p < 0.001; SR141716A-hGMSCs vs. AM630-hGMSCs ***p < 0.001.
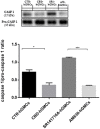
Figure 3
Western blot analysis for CASP1. CASP1 expression is decreased in CBD-hGMSCs compared to CTR-DMSO). The levels of CASP1 are significant increase in SR141716A-hGMSCs compared with CBD-hGMSCs. CTR-hGMSCs vs. CBD-hGMSCs *p < 0.05; SR141716A-hGMSCs vs. AM630-hGMSCs ***p < 0.001.
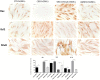
Figure 4
Immunocytochemical staining for Bax, Bcl2 and NFκB. CBD-hGMSCs showed a negative staining for Bax, a reduced expression for NF-KB and a positive staining for Bcl2, compared to CTR-hGMSCs. hGMSCs-SR141716A showed a marked positive cytoplasmatic/nuclear staining for NF-kB, as indicated by the arrows. Moreover, hGMSCs-SR141716A showed an increased nuclear expression for Bax and reduced expression for Bcl2 at both cytoplasmatic and nuclear compartment, compared to CBD-hGMSCs. Instead, hGMSCs-AM630 showed a reduced expression for Bax and Bcl2 compared to CBD-hGMSCs. Whereas, no differences statistically differences were found for the NF-kB expression between CBD-hGMSCs and hGMSCs-AM630. The graph represented the densytometric quantitative analysis. For Bax CTR-hGMSCs vs. CBD-hGMSCs ***p < 0.001; SR141716A-hGMSCs vs. AM630-hGMSCs ****p < 0.0001. For Bcl2 CTR-hGMSCs vs. CBD-hGMSCs *p < 0.05; SR141716A-hGMSCs vs. AM630-hGMSCs ***p < 0.001. For NF-kB CTR-hGMSCs vs. CBD-hGMSCs *p < 0.05; SR141716A-hGMSCs vs. AM630-hGMSCs ****p < 0.0001.
Figure 5
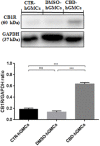
Western blot analysis for CB1R. CB1R expression is increased in CBD-hGMSCs compared to control groups (DMSO-hGMSCs and CTR-hGMSCs). ***p < 0.001. ns, no statistical differences.
Figure 6
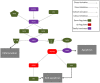
Proposed molecular mechanism for CBD modulation of the genes of the NALP3-inflammasome in hGMSCs. The activation of the NALP3-inflammasome is regulated by NF-kB which promotes NALP3 deubiquitination via activation of BRCC36. NALP3 interacts with CASP1 through CARD5 and CARD8. Activated CASP1 cleaves pro-IL-1 beta and pro-IL-18, leading to subsequent release of mature cytokines which trigger inflammation. In parallel, IL-18 indirectly via CASP8 and CASP6 can stimulate apoptosis. In hGMSCs, BRCC6, NALP3 and CASP1 transcripts are suppressed (violet) and the expression of the downstream mediators (CARD5, CARD8, IL1β, IL18, CASP8) is downregulated (green), while CASP8 is totally suppressed (violet). Instead, the pro-survival AKT1 and MDM2 were upregulated (red), leading to the downregulation of BAX and BAD (green).
Similar articles
- Transcriptomic Analysis of Stem Cells Treated with Moringin or Cannabidiol: Analogies and Differences in Inflammation Pathways.
Chiricosta L, Silvestro S, Pizzicannella J, Diomede F, Bramanti P, Trubiani O, Mazzon E. Chiricosta L, et al. Int J Mol Sci. 2019 Nov 30;20(23):6039. doi: 10.3390/ijms20236039. Int J Mol Sci. 2019. PMID: 31801206 Free PMC article. - Cannabidiol Activates Neuronal Precursor Genes in Human Gingival Mesenchymal Stromal Cells.
Soundara Rajan T, Giacoppo S, Scionti D, Diomede F, Grassi G, Pollastro F, Piattelli A, Bramanti P, Mazzon E, Trubiani O. Soundara Rajan T, et al. J Cell Biochem. 2017 Jun;118(6):1531-1546. doi: 10.1002/jcb.25815. Epub 2016 Dec 29. J Cell Biochem. 2017. PMID: 27918106 - Cannabidiol Modulates the Expression of Alzheimer's Disease-Related Genes in Mesenchymal Stem Cells.
Libro R, Diomede F, Scionti D, Piattelli A, Grassi G, Pollastro F, Bramanti P, Mazzon E, Trubiani O. Libro R, et al. Int J Mol Sci. 2016 Dec 23;18(1):26. doi: 10.3390/ijms18010026. Int J Mol Sci. 2016. PMID: 28025562 Free PMC article. - In Vitro Studies on Therapeutic Effects of Cannabidiol in Neural Cells: Neurons, Glia, and Neural Stem Cells.
Kim J, Choi H, Kang EK, Ji GY, Kim Y, Choi IS. Kim J, et al. Molecules. 2021 Oct 8;26(19):6077. doi: 10.3390/molecules26196077. Molecules. 2021. PMID: 34641624 Free PMC article. Review. - Cannabidiol as a Novel Therapeutic for Immune Modulation.
Peyravian N, Deo S, Daunert S, Jimenez JJ. Peyravian N, et al. Immunotargets Ther. 2020 Aug 18;9:131-140. doi: 10.2147/ITT.S263690. eCollection 2020. Immunotargets Ther. 2020. PMID: 32903924 Free PMC article. Review.
Cited by
- MicroRNA 210 Mediates VEGF Upregulation in Human Periodontal Ligament Stem Cells Cultured on 3DHydroxyapatite Ceramic Scaffold.
Pizzicannella J, Cavalcanti M, Trubiani O, Diomede F. Pizzicannella J, et al. Int J Mol Sci. 2018 Dec 6;19(12):3916. doi: 10.3390/ijms19123916. Int J Mol Sci. 2018. PMID: 30563289 Free PMC article. - Cannabidiol selectively modulates interleukin (IL)-1β and IL-6 production in toll-like receptor activated human peripheral blood monocytes.
Sermet S, Li J, Bach A, Crawford RB, Kaminski NE. Sermet S, et al. Toxicology. 2021 Dec;464:153016. doi: 10.1016/j.tox.2021.153016. Epub 2021 Nov 2. Toxicology. 2021. PMID: 34740670 Free PMC article. - P2X7 receptors from the perspective of NLRP3 inflammasome pathway in depression: Potential role of cannabidiol.
Akcay E, Karatas H. Akcay E, et al. Brain Behav Immun Health. 2024 Sep 3;41:100853. doi: 10.1016/j.bbih.2024.100853. eCollection 2024 Nov. Brain Behav Immun Health. 2024. PMID: 39296605 Free PMC article. - Transcriptomic Analysis of Stem Cells Treated with Moringin or Cannabidiol: Analogies and Differences in Inflammation Pathways.
Chiricosta L, Silvestro S, Pizzicannella J, Diomede F, Bramanti P, Trubiani O, Mazzon E. Chiricosta L, et al. Int J Mol Sci. 2019 Nov 30;20(23):6039. doi: 10.3390/ijms20236039. Int J Mol Sci. 2019. PMID: 31801206 Free PMC article. - The Anti-Inflammatory Effects of Cannabidiol (CBD) on Acne.
Peyravian N, Deo S, Daunert S, Jimenez JJ. Peyravian N, et al. J Inflamm Res. 2022 May 3;15:2795-2801. doi: 10.2147/JIR.S355489. eCollection 2022. J Inflamm Res. 2022. PMID: 35535052 Free PMC article. Review.
References
- Bauernfeind F. G., Horvath G., Stutz A., Alnemri E. S., MacDonald K., Speert D., et al. . (2009). Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791. 10.4049/jimmunol.0901363 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous