Regulation of Hippo pathway transcription factor TEAD by p38 MAPK-induced cytoplasmic translocation - PubMed (original) (raw)
Regulation of Hippo pathway transcription factor TEAD by p38 MAPK-induced cytoplasmic translocation
Kimberly C Lin et al. Nat Cell Biol. 2017.
Erratum in
- Author Correction: Regulation of Hippo pathway transcription factor TEAD by p38 MAPK-induced cytoplasmic translocation.
Lin KC, Moroishi T, Meng Z, Jeong HS, Plouffe SW, Sekido Y, Han J, Park HW, Guan KL. Lin KC, et al. Nat Cell Biol. 2018 Sep;20(9):1098. doi: 10.1038/s41556-018-0101-8. Nat Cell Biol. 2018. PMID: 30018319
Abstract
The Hippo pathway controls organ size and tissue homeostasis, with deregulation leading to cancer. The core Hippo components in mammals are composed of the upstream serine/threonine kinases Mst1/2, MAPK4Ks and Lats1/2. Inactivation of these upstream kinases leads to dephosphorylation, stabilization, nuclear translocation and thus activation of the major functional transducers of the Hippo pathway, YAP and its paralogue TAZ. YAP/TAZ are transcription co-activators that regulate gene expression primarily through interaction with the TEA domain DNA-binding family of transcription factors (TEAD). The current paradigm for regulation of this pathway centres on phosphorylation-dependent nucleocytoplasmic shuttling of YAP/TAZ through a complex network of upstream components. However, unlike other transcription factors, such as SMAD, NF-κB, NFAT and STAT, the regulation of TEAD nucleocytoplasmic shuttling has been largely overlooked. In the present study, we show that environmental stress promotes TEAD cytoplasmic translocation via p38 MAPK in a Hippo-independent manner. Importantly, stress-induced TEAD inhibition predominates YAP-activating signals and selectively suppresses YAP-driven cancer cell growth. Our data reveal a mechanism governing TEAD nucleocytoplasmic shuttling and show that TEAD localization is a critical determinant of Hippo signalling output.
Conflict of interest statement
Competing financial interests
K.-L.G. is a co-founder and has an equity interest in Vivace Therapeutics, Inc. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies.
Figures
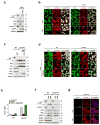
Figure 1. p38-mediates stress-induced TEAD cytoplasmic translocation
a, Immunofluorescence staining of TEAD and YAP/TAZ in HEK293A cells treated with YAP-inhibiting signals. b, Immunofluorescence detects TEAD cytoplasmic translocation by environmental stress. c, Effect of p38 inhibitors on osmotic stress induced-TEAD cytoplasmic translocation. HEK293A cells were pretreated with p38 inhibitors, and stimulated with NaCl and stained for immunofluorescence. d, Ectopic expression of MKK3/p38 promotes TEAD4 cytoplasmic translocation. 24 hr after transfection, cells were treated with p38 inhibitors for 8hr and stained for immunofluorescence. e, Immunofluorescence staining shows deletion of p38 impairs TEAD nucleocytoplasmic shuttling by osmotic stress. Data for two independent p38 4KO clones are shown. f, Western blotting of p38 isoforms in p38 4KO cells. g, p38 mediates inhibition of YAP-TEAD target gene expression by stress. WT and TEAD KO cells were pretreated with NaCl and SB203580 as indicated, and then stimulated with 10% serum. CTGF mRNA expression was measured by qRT-PCR. Data are presented as mean ± s.e.m. from n=3 independent experiments. h, Osmotic stress inhibits YAP-TEAD target gene expression. Cells were subject to serum starvation or NaCl, and then LPA-induced CTGF and CYR61 mRNA expression was measured by qRT-PCR. Data are presented as mean ± s.e.m. from n=3 independent experiments. i, Correlation between stress-induced cytoplasmic translocation of TEAD and p38. Cells were stimulated with NaCl for the indicated times and then subjected to immunofluorescence to detect p38. Quantification of TEAD nuclear localization (N) and cytoplasmic localization (C) is provided. Random views (~100 cells) were selected for quantification. j, Time course of p38 activation by NaCl. Western blotting of phospho-p38 and its substrate phospho-MK2 upon NaCl treatment. k, Inverse correlation between stress-induced cytoplasmic translocation of TEAD and phospho-p38. Cells were stimulated with NaCl for 1 hr and then subjected to immunofluorescence using a phospho-p38 antibody. Scale bars in a–e, i, and k are 20μm. Statistics source data are shown in Supplementary Table 1. Unprocessed scans of blots are shown in Supplementary Figure 5.
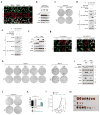
Figure 2. p38 mediates stress-induced TEAD cytoplasmic translocation via protein-protein interaction
a, Detection of osmotic stress-induced TEAD4 and p38 interaction by immunoprecipitation (IP) assay. IP and WB denote immunoprecipitation and Western blot, respectively. b, Immunoprecipitation showing time course of sorbitol-induced TEAD-p38 interaction. c, TEAD immunoprecipitation shows osmotic stress ablates TEAD-YAP interaction. d, TEAD interacts with p38 and MKK3 as shown by immunoprecipitation. e, In vitro binding assay using bacterially-purified proteins shows a direct interaction between p38 and TEAD. f, p38 does not interact with YAP. g, Sequence alignment of TEAD with canonical p38 substrates. Putative D domain (red) is conserved in N-terminus of all TEAD isoforms. h, The TEAD D domain is required for interaction with p38. p38 binds TEAD4 C-terminal truncation constructs (1–339 and 1–382), but not N-terminal truncations (120–434 or 181–434) in an immunoprecipitation assay. i–k, The p38 CD/ED docking motif is required for interaction with TEAD. p38 WT, but not p38 CD/ED mutant co-immunoprecipitates with TEAD1 (g), TEAD2 (h), and TEAD4 (i). l, TEAD-p38 interaction mediates TEAD cytoplasmic translocation. Immunofluorescence staining shows TEAD cytoplasmic translocation occurs in cells transfected with p38 WT, but not CD/ED mutant. m, Effect of p38 kinase activity on p38-TEAD binding. Immunoprecipitation assay shows TEAD binds p38 WT but not kinase-dead mutant, p38KM. n, Effect of p38 kinase activity on TEAD cytoplasmic translocation. Immunofluorescence shows ectopic expression of p38 WT, but not kinase-dead mutant p38 KM, induces TEAD4 cytoplasmic translocation. Scale bars in l and n are 20μm. Unprocessed scans of blots are shown in Supplementary Figure 5.
Figure 3. TEAD cytoplasmic translocation prevents YAP activation
a, b, Effect of osmotic stress on serum-, and LPA-induced YAP activation. Osmotic stress blocks serum and LPA-induced YAP dephosphorylation as shown by Western blot (a, lower arrow), and nuclear translocation as shown by immunofluorescence staining (b). c, d, Stress-induced TEAD and YAP cytoplasmic translocation is p38-dependent, but Hippo-independent. YAP is constitutively dephosphorylated in Lats KO cells as indicated by YAP phostag gel (c). Immunofluorescence shows stress induces YAP and TEAD cytoplasmic translocation in the Lats KO cells, which is blocked by SB203580 treatment (d). e, Quantification of CTGF mRNA by qRT-PCR in WT and Lats KO cells stimulated with osmotic stress with or without SB203580 treatment. Data are presented as mean ± s.e.m. from n=3 independent experiments. f, g, Detection of YAP/TAZ localization by immunofluorescence staining in TEAD KO cells stimulated with LPA or serum. Western blotting indicates YAP dephosphorylation by LPA or serum stimulation is intact in TEAD KO cells (f), whereas immunofluorescence shows YAP nuclear accumulation is impaired (g). Scale bars in b, d, and g are 20μm. Statistics source data are shown in Supplementary Table 1. Unprocessed scans of blots are shown in Supplementary Figure 5.
Figure 4. TEAD inhibition restricts YAP-driven cancer cell growth
a, b, Effect of osmotic stress on TEAD and YAP cytoplasmic sequestration in MSTO-211H mesothelioma cells. Note that unlike HEK293A (Fig. 1a), serum starvation, glucose starvation, and latruculin B did not induce YAP cytoplasmic localization because of Lats2 mutation in MSTO-211H cells. Only NaCl treatment elicited TEAD and YAP cytoplasmic translocation as detected by immunofluorescence (a) despite constitutive YAP dephosphorylation as detected by Western blot (b). c, Stress-induced TEAD inhibition suppresses anchorage independent growth. Osmotic stress inhibited colony formation in control, but not TEAD1/4-VP16 expressing MSTO-211H cells. d, e, Immunoprecipitation shows p38 cannot bind TEAD1-VP16 (d) or TEAD4-VP16 (e). f, Western blotting of YAP phosphorylation status in UM cell lines 92.1 and OCM1. g, Immunostaining of TEAD and YAP/TAZ in UM cell lines 92.1 and OCM1 upon osmotic stress. Note that YAP displays cytoplasmic staining under normal condition in OCM1 cells. Scale bars in a and g are 20μm. h, Differential effect of TEAD inhibition on anchorage-independent growth of GNAQ-mutant and BRAF-mutant UM cells. Stress-induced TEAD inhibition ablated colony formation in all GNAQ-mutant cell lines, whereas BRAF-mutant cell growth was insensitive. i, Western blot for PARP cleavage in 92.1 and OCM1 cells. NaCl stimulation induces apoptosis in 92.1 but not OCM1. Pretreatment with p38 inhibitor rescues cells from osmotic stress-induced apoptosis. j, TEAD1/4 -VP16 rescues p38-induced inhibition of colony formation of MSTO-211H cells. k, Quantification of (j). n=3 biological replicates. Data are presented as mean ± s.e.m. *p < 0.05; **p < 0.01; p values were determined using one-way ANOVA followed by Tukey’s multiple comparison test. l, TEAD1/4-VP16 rescues p38-induced inhibition of MSTO-211H in vivo tumor xenograft growth. Nude mice were injected with control, p38, or p38 + TEAD1/4-VP16 expressing MSTO-211H cells and tumor growth was measured at the indicated times. Data are presented as mean ± s.e.m. n = 4 mice per group. **p < 0.01; ***p < 0.001; p values were determined using two-way ANOVA. m, Nude mice were injected with control, p38, or p38 + TEAD1/4-VP16 expressing MSTO-211H cells and tumors were harvested after 4 weeks. Only three tumors developed in the p38 group. Scale bar, 10mm.
Similar articles
- Distinct effects of Hippo-YAP/TAZ and YAP/TAZ-TEAD in epithelial maintenance and repair.
Yuan Q, Yuan Y, Peng Y, Xia X, Chen Q, Yu FX, Feng X. Yuan Q, et al. Biochem Biophys Res Commun. 2025 Mar 5;751:151427. doi: 10.1016/j.bbrc.2025.151427. Epub 2025 Jan 30. Biochem Biophys Res Commun. 2025. PMID: 39903968 - Hippo pathway effectors YAP, TAZ and TEAD are associated with EMT master regulators ZEB, Snail and with aggressive phenotype in phyllodes breast tumors.
Akrida I, Makrygianni M, Nikou S, Mulita F, Bravou V, Papadaki H. Akrida I, et al. Pathol Res Pract. 2024 Oct;262:155551. doi: 10.1016/j.prp.2024.155551. Epub 2024 Aug 15. Pathol Res Pract. 2024. PMID: 39153238 - Nuclear phosphoinositide signaling promotes YAP/TAZ-TEAD transcriptional activity in breast cancer.
Jung O, Baek MJ, Wooldrik C, Johnson KR, Fisher KW, Lou J, Ricks TJ, Wen T, Best MD, Cryns VL, Anderson RA, Choi S. Jung O, et al. EMBO J. 2024 May;43(9):1740-1769. doi: 10.1038/s44318-024-00085-6. Epub 2024 Apr 2. EMBO J. 2024. PMID: 38565949 Free PMC article. - Hippo pathway inhibition by blocking the YAP/TAZ-TEAD interface: a patent review.
Crawford JJ, Bronner SM, Zbieg JR. Crawford JJ, et al. Expert Opin Ther Pat. 2018 Dec;28(12):867-873. doi: 10.1080/13543776.2018.1549226. Epub 2018 Dec 2. Expert Opin Ther Pat. 2018. PMID: 30482112 Review. - Reciprocal regulation of YAP/TAZ by the Hippo pathway and the Small GTPase pathway.
Jang JW, Kim MK, Bae SC. Jang JW, et al. Small GTPases. 2020 Jul;11(4):280-288. doi: 10.1080/21541248.2018.1435986. Epub 2018 Apr 20. Small GTPases. 2020. PMID: 29457552 Free PMC article. Review.
Cited by
- Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine.
Dey A, Varelas X, Guan KL. Dey A, et al. Nat Rev Drug Discov. 2020 Jul;19(7):480-494. doi: 10.1038/s41573-020-0070-z. Epub 2020 Jun 17. Nat Rev Drug Discov. 2020. PMID: 32555376 Free PMC article. Review. - Comparative transcriptomic analysis validates iPSC derived in-vitro progressive fibrosis model as a screening tool for drug discovery and development in systemic sclerosis.
Nathan S, Wang Y, D'ambrosio M, Paul R, Lyu H, Delic D, Bretschneider T, Falana K, Li L, Vijayaraj P. Nathan S, et al. Sci Rep. 2024 Oct 18;14(1):24428. doi: 10.1038/s41598-024-74610-2. Sci Rep. 2024. PMID: 39424619 Free PMC article. - The Hippo Signaling Network and Its Biological Functions.
Misra JR, Irvine KD. Misra JR, et al. Annu Rev Genet. 2018 Nov 23;52:65-87. doi: 10.1146/annurev-genet-120417-031621. Epub 2018 Sep 5. Annu Rev Genet. 2018. PMID: 30183404 Free PMC article. Review. - The Hippo pathway drives the cellular response to hydrostatic pressure.
Park J, Jia S, Salter D, Bagnaninchi P, Hansen CG. Park J, et al. EMBO J. 2022 Jul 4;41(13):e108719. doi: 10.15252/embj.2021108719. Epub 2022 Jun 15. EMBO J. 2022. PMID: 35702882 Free PMC article. - The HIPPO pathway in gynecological malignancies.
Wang D, He J, Dong J, Meyer TF, Xu T. Wang D, et al. Am J Cancer Res. 2020 Feb 1;10(2):610-629. eCollection 2020. Am J Cancer Res. 2020. PMID: 32195031 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- R01 DE015964/DE/NIDCR NIH HHS/United States
- R01 GM051586/GM/NIGMS NIH HHS/United States
- R35 CA196878/CA/NCI NIH HHS/United States
- T32 GM007752/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous