The Role of Interferons in Inflammation and Inflammasome Activation - PubMed (original) (raw)
Review
The Role of Interferons in Inflammation and Inflammasome Activation
Nataša Kopitar-Jerala. Front Immunol. 2017.
Abstract
Inflammation is an essential physiological process, which enables survival during infection and maintains tissue homeostasis. Interferons (IFNs) and pro- and anti-inflammatory cytokines are crucial for appropriate response to pathogens, damaged cells, or irritants in inflammatory response. The inflammasom is multiprotein complex, which initiates cleavage of pro-inflammatory cytokines IL-1β and IL-18 into active forms. In addition, inflammasomes initiate pyroptotic cell death. In the present review, I summarize and analyze recent findings regarding the cross talk of IFNs and inflammasomes.
Keywords: caspase-1; caspase-11; cyclic GMP-AMP synthase; guanylate-binding protein; inflammasome; interferon; macrophages; pyropotosis.
Figures
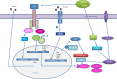
Figure 1
Type I interferons (IFNs) and inflammasome activation. Initial pathogen-associated molecular patterns (PAMPs) recognition by pattern recognition receptors induces IFN-β expression. IFNs could signal in an autocrine or paracrine manner and trigger expression of IFN-stimulated genes (ISGs): interferon regulatory factor (IRF)1, AIM2, caspase-11. Caspase-11 recognizes cytosolic LPS and induces IL-1β processing in an Nlrp3-dependent manner and triggers pyroptosis through gasdermin D (GSDMD) cleavage. Active caspase-1 and caspase-11 cleave GSDMD and the released gasdermin-N domain binds to phosphoinositides in the plasma membrane, oligomerizes to generate membrane pores, and initiates cell death-pyroptosis. IRF induce the expression of guanylate-binding proteins (GBPs), which target vacuolar and cytosolic bacteria, compromise the integrity of bacterial cells, and expose PAMPs like LPS and dsDNA to cytosolic sensors, caspase-11, and AIM2. IFN signaling triggers the expression of inducible nitric oxide synthase (iNOS), which upregulates cellular nitric oxide (NO) levels leading to NLRP3 S-nitrosylation.
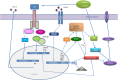
Figure 2
Interferon (IFN) signaling influence recognition of intracellular pathogens—cytosolic bacteria and influenza A virus (IAV). IFNs signaling trigger the transcription factor interferon regulatory factor (IRF)1, which promotes expression of guanylate-binding proteins (GBPs) and interferon response gene B10 (IRGB10). IRGB10, together with GBPs permeabilizes the membrane of Gram-negative bacteria, an action that results in release of bacterial DNA and LPS. Bacterial cytosolic DNA is sensed by Aim2 inflammasome and LPS directly interacts with caspase-11. Type I IFN signaling mediates upregulation of interferon-inducible protein Z-DNA-binding protein 1 (ZBP1), which recognizes the IAV proteins and triggers NLRP3 inflammasome activation, as well as induction of apoptosis, necroptosis, and pyroptosis in IAV-infected cells.
Similar articles
- Guanylate-Binding Protein-Dependent Noncanonical Inflammasome Activation Prevents Burkholderia thailandensis-Induced Multinucleated Giant Cell Formation.
Dilucca M, Ramos S, Shkarina K, Santos JC, Broz P. Dilucca M, et al. mBio. 2021 Aug 31;12(4):e0205421. doi: 10.1128/mBio.02054-21. Epub 2021 Aug 17. mBio. 2021. PMID: 34399626 Free PMC article. - Interferons and inflammasomes: Cooperation and counterregulation in disease.
Labzin LI, Lauterbach MA, Latz E. Labzin LI, et al. J Allergy Clin Immunol. 2016 Jul;138(1):37-46. doi: 10.1016/j.jaci.2016.05.010. Epub 2016 May 24. J Allergy Clin Immunol. 2016. PMID: 27373324 Review. - Uncoupled pyroptosis and IL-1β secretion downstream of inflammasome signaling.
Li Y, Jiang Q. Li Y, et al. Front Immunol. 2023 Apr 6;14:1128358. doi: 10.3389/fimmu.2023.1128358. eCollection 2023. Front Immunol. 2023. PMID: 37090724 Free PMC article. Review. - Pyroptotic and non-pyroptotic effector functions of caspase-11.
Abu Khweek A, Amer AO. Abu Khweek A, et al. Immunol Rev. 2020 Sep;297(1):39-52. doi: 10.1111/imr.12910. Epub 2020 Aug 1. Immunol Rev. 2020. PMID: 32737894 Free PMC article. Review. - The ATP-P2X7 Signaling Axis Is an Essential Sentinel for Intracellular Clostridium difficile Pathogen-Induced Inflammasome Activation.
Liu YH, Chang YC, Chen LK, Su PA, Ko WC, Tsai YS, Chen YH, Lai HC, Wu CY, Hung YP, Tsai PJ. Liu YH, et al. Front Cell Infect Microbiol. 2018 Mar 16;8:84. doi: 10.3389/fcimb.2018.00084. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29616195 Free PMC article.
Cited by
- Influence of Donor-Specific Characteristics on Cytokine Responses in H3N2 Influenza A Virus Infection: New Insights from an Ex Vivo Model.
Huang CG, Hsieh MJ, Wu YC, Huang PW, Lin YJ, Tsao KC, Shih SR, Lee LA. Huang CG, et al. Int J Mol Sci. 2024 Oct 11;25(20):10941. doi: 10.3390/ijms252010941. Int J Mol Sci. 2024. PMID: 39456722 Free PMC article. - IRF3 function and immunological gaps in sepsis.
Basak B, Akashi-Takamura S. Basak B, et al. Front Immunol. 2024 Feb 5;15:1336813. doi: 10.3389/fimmu.2024.1336813. eCollection 2024. Front Immunol. 2024. PMID: 38375470 Free PMC article. Review. - Vitamin D3/VDR inhibits inflammation through NF-κB pathway accompanied by resisting apoptosis and inducing autophagy in abalone Haliotis discus hannai.
Huang D, Guo Y, Li X, Pan M, Liu J, Zhang W, Mai K. Huang D, et al. Cell Biol Toxicol. 2023 Jun;39(3):885-906. doi: 10.1007/s10565-021-09647-4. Epub 2021 Oct 12. Cell Biol Toxicol. 2023. PMID: 34637036 - Editorial: Regulation of the phenotype and function of human macrophages and dendritic cells by exogenous immunomodulators.
Bazzi S, Bahr GM, Lampiasi N. Bazzi S, et al. Front Immunol. 2023 Dec 19;14:1353765. doi: 10.3389/fimmu.2023.1353765. eCollection 2023. Front Immunol. 2023. PMID: 38193089 Free PMC article. No abstract available. - A Synergic Strategy: Adipose-Derived Stem Cell Spheroids Seeded on 3D-Printed PLA/CHA Scaffolds Implanted in a Bone Critical-Size Defect Model.
Kronemberger GS, Palhares TN, Rossi AM, Verçosa BRF, Sartoretto SC, Resende R, Uzeda MJ, Alves ATNN, Alves GG, Calasans-Maia MD, Granjeiro JM, Baptista LS. Kronemberger GS, et al. J Funct Biomater. 2023 Nov 21;14(12):555. doi: 10.3390/jfb14120555. J Funct Biomater. 2023. PMID: 38132809 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous