Regulation of the Hippo pathway in cancer biology - PubMed (original) (raw)
Review
Regulation of the Hippo pathway in cancer biology
Sungho Moon et al. Cell Mol Life Sci. 2018 Jul.
Abstract
The Hippo tumor suppressor pathway, which is well conserved from Drosophila to humans, has emerged as the master regulator of organ size, as well as major cellular properties, such as cell proliferation, survival, stemness, and tissue homeostasis. The biological significance and deregulation of the Hippo pathway in tumorigenesis have received a surge of interest in the past decade. In the current review, we present the major discoveries that made substantial contributions to our understanding of the Hippo pathway and discuss how Hippo pathway components contribute to cellular signaling, physiology, and their potential implications in anticancer therapeutics.
Keywords: Cancer; Hippo pathway; TEAD; Therapeutic target; YAP/TAZ.
Figures
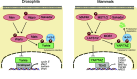
Fig. 1
Core Hippo pathway components in Drosophila and mammals. In Drosophila (left), Hippo (Hpo) and Misshapen (Msn) kinase phosphorylate and activate Warts (Wts) kinase, which in turn inactivate the transcriptional co-activator Yorkie (Yki). Phosphorylation of Yki leads to cytoplasmic retention via a 14-3-3 interaction. Upon Hippo pathway inactivation, dephosphorylated Yki translocates to the nucleus and binds the transcription factor Scalloped (Sd) to induce gene expression involved in cell proliferation and anti-apoptosis. In mammals (right), the core Hippo pathway components are evolutionarily conserved. MST1/2 kinase phosphorylates LATS1/2, which in turn phosphorylates and inhibits YAP/TAZ. Phosphorylation of YAP/TAZ leads to cytoplasmic sequestration and proteasomal degradation
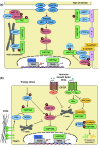
Fig. 2
Regulators and regulations of the Hippo pathway. Hippo pathway components in mammals are shown in various colors. Pointed arrows indicate activation, and blunt-ended lines indicate inhibition. Hippo cascade kinases are shown in red and inhibitory regulators of YAP/TAZ activity are shown in blue. a Hippo pathway is regulated by cell polarity (Crumbs, DACH1–FAT4) and cell–cell junctions (adherens junction, tight junction). AMOT angiomotin, AJ adherens junction, CRB Crumbs homolog, DACH1 Dachous-1, FRMD6 FERM domain-containing protein 6, LATS large tumor suppressor homolog, MST mammalian STE20-like protein kinase, NF2 neurofibromin 2 (also known as Merlin), PTPN14 protein tyrosine phosphatase, non-receptor type 14, SCRIB scribbled planar-cell polarity protein, TAZ transcriptional co-activator with PDZ-binding motif, TJ tight junction, VGLL4 vestigial-like protein 4, YAP Yes-associated protein, ZO zona occludens protein. b Hippo pathway is regulated by extracellular ligands, stress responses, and mechanotransduction. AMPK 5′ AMP-activated protein kinase, APC adenomatous polyposis coli, β-TRCP β-transducin repeat-containing E3 ubiquitin protein ligase, ECM extracellular matrix, GPCR G protein-coupled receptor, Rho Ras homolog gene family, ROCK Rho-associated protein kinase
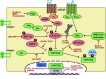
Fig. 3
Putative targets for therapeutic intervention in the Hippo pathway. Summary of the positive and negative regulators of Hippo kinases and YAP/TAZ, respectively. Activating components’ upstream of YAP/TAZ is in green, and inhibitory components are in red
Similar articles
- The Role of Hippo Pathway in Cancer Stem Cell Biology.
Park JH, Shin JE, Park HW. Park JH, et al. Mol Cells. 2018 Feb 28;41(2):83-92. doi: 10.14348/molcells.2018.2242. Epub 2018 Feb 5. Mol Cells. 2018. PMID: 29429151 Free PMC article. Review. - The regulation and function of YAP transcription co-activator.
Zhu C, Li L, Zhao B. Zhu C, et al. Acta Biochim Biophys Sin (Shanghai). 2015 Jan;47(1):16-28. doi: 10.1093/abbs/gmu110. Epub 2014 Dec 8. Acta Biochim Biophys Sin (Shanghai). 2015. PMID: 25487920 Review. - The Hippo signaling pathway in liver regeneration and tumorigenesis.
Hong L, Cai Y, Jiang M, Zhou D, Chen L. Hong L, et al. Acta Biochim Biophys Sin (Shanghai). 2015 Jan;47(1):46-52. doi: 10.1093/abbs/gmu106. Epub 2014 Dec 4. Acta Biochim Biophys Sin (Shanghai). 2015. PMID: 25476204 Review. - Hippo signaling in stress response and homeostasis maintenance.
Mao B, Gao Y, Bai Y, Yuan Z. Mao B, et al. Acta Biochim Biophys Sin (Shanghai). 2015 Jan;47(1):2-9. doi: 10.1093/abbs/gmu109. Epub 2014 Dec 4. Acta Biochim Biophys Sin (Shanghai). 2015. PMID: 25476206 Review. - Hippo pathway in mammary gland development and breast cancer.
Shi P, Feng J, Chen C. Shi P, et al. Acta Biochim Biophys Sin (Shanghai). 2015 Jan;47(1):53-9. doi: 10.1093/abbs/gmu114. Epub 2014 Dec 2. Acta Biochim Biophys Sin (Shanghai). 2015. PMID: 25467757 Review.
Cited by
- Hippo pathway activation mediates chemotherapy-induced anti-cancer effect and cardiomyopathy through causing mitochondrial damage and dysfunction.
She G, Du JC, Wu W, Pu TT, Zhang Y, Bai RY, Zhang Y, Pang ZD, Wang HF, Ren YJ, Sadoshima J, Deng XL, Du XJ. She G, et al. Theranostics. 2023 Jan 1;13(2):560-577. doi: 10.7150/thno.79227. eCollection 2023. Theranostics. 2023. PMID: 36632235 Free PMC article. - microRNAs Promoting Growth of Gastric Cancer Xenografts and Correlation to Clinical Prognosis.
Weidle UH, Birzele F, Nopora A. Weidle UH, et al. Cancer Genomics Proteomics. 2021 Jan-Feb;18(1):1-15. doi: 10.21873/cgp.20237. Epub 2021 Jan 8. Cancer Genomics Proteomics. 2021. PMID: 33419892 Free PMC article. Review. - Transposon Mutagenesis-Guided CRISPR/Cas9 Screening Strongly Implicates Dysregulation of Hippo/YAP Signaling in Malignant Peripheral Nerve Sheath Tumor Development.
Vélez-Reyes GL, Koes N, Ryu JH, Kaufmann G, Berner M, Weg MT, Wolf NK, Rathe SK, Ratner N, Moriarity BS, Largaespada DA. Vélez-Reyes GL, et al. Cancers (Basel). 2021 Mar 30;13(7):1584. doi: 10.3390/cancers13071584. Cancers (Basel). 2021. PMID: 33808166 Free PMC article. - Deletion of Nf2 in neural crest-derived tongue mesenchyme alters tongue shape and size, Hippo signalling and cell proliferation in a region- and stage-specific manner.
Ishan M, Chen G, Yu W, Wang Z, Giovannini M, Cao X, Liu HX. Ishan M, et al. Cell Prolif. 2021 Dec;54(12):e13144. doi: 10.1111/cpr.13144. Epub 2021 Oct 26. Cell Prolif. 2021. PMID: 34697858 Free PMC article. - New Insights into YES-Associated Protein Signaling Pathways in Hematological Malignancies: Diagnostic and Therapeutic Challenges.
Allegra A, Pioggia G, Innao V, Musolino C, Gangemi S. Allegra A, et al. Cancers (Basel). 2021 Apr 20;13(8):1981. doi: 10.3390/cancers13081981. Cancers (Basel). 2021. PMID: 33924049 Free PMC article. Review.
References
- Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121(4):1053–1063. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous