Antitumor Effects of Berberine on Gliomas via Inactivation of Caspase-1-Mediated IL-1β and IL-18 Release - PubMed (original) (raw)
Antitumor Effects of Berberine on Gliomas via Inactivation of Caspase-1-Mediated IL-1β and IL-18 Release
Lei Tong et al. Front Oncol. 2019.
Abstract
Gliomas arise in the glial cells of the brain or spine and are the most prevalent and devastating type of brain tumors. Studies of tumor immunology have established the importance of the tumor micro-environment as a driver of oncogenesis. Inflammatory mediators such as IL-1β and IL-18 released by monocytes regulate transcriptional networks that are required for malignant cell growth. Berberine is a natural botanical alkaloid that is widely found in the Berberis species. Although it has been widely used as an anti-diarrheal treatment in North America for several decades, our study is the first to investigate berberine as an anti-tumor agent in glioma cells. In this study, we demonstrate that berberine significantly inhibits inflammatory cytokine Caspase-1 activation via ERK1/2 signaling and subsequent production of IL-1β and IL-18 by glioma cells. Moreover, we found that berberine treatment led to decreased motility and subsequently cell death in U251 and U87 cells. In addition, our study is the first to indicate that berberine can reverse the process of epithelial-mesenchymal transition, a marker of tumor invasion. Taken together, our work supports berberine as a putative anti-tumor agent targeting glioma cells.
Keywords: EMT; ERK1/2; IL-18; IL-1β; berberine; gliomas.
Figures
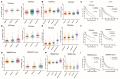
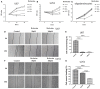
Figure 1
Associations of IL-1β and IL-18 expression with clinical and molecular characteristics in gliomas. IL-18 and IL-1β mRNA level was significantly increased in Grade IV gliomas as compared to Grade II. The results were obtained from TCGA (A), CGGA (D) and REMBRANDT (G) cohorts, respectively. IL-18 and IL-1β mRNA levels were significantly increased in mesenchymal gliomas as compared to proneural gliomas. The results were obtained from TCGA (B), CGGA (E), and REMBRANDT (H) cohorts, respectively. Kaplan meier method was applied to compute overall median survival between patients with high expression of IL-18, IL-1β and low expression of IL-18, IL-1β. The data was collected from TCGA (C), CGGA (F), and REMBRANDT (I) cohorts, respectively.
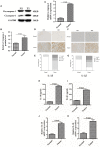
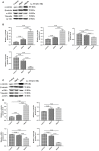
Figure 2
The expression of caspase-1, IL-18, and IL-1β were significantly upregulated in glioma than normal brain tissues. (A) Protein levels of caspase-1 and cleaved caspase-1 in normal brain tissues and gliomas. (B) Protein levels of cleaved caspase-1 was quantified by densitometric analysis using NIH ImageJ, normalized to β-actin and presented as fold changes compared to the first lane (arbitrarily set at a value of 1). (C) mRNA levels of caspase-1 between normal brain tissues and gliomas were compared by qPCR. Unpaired student _t_-test was applied for statistical analysis, p < 0.05 was considered as statistically significant. (D,F) IHC staining of IL-1β and IL-18 in normal brain tissues and gliomas. The pictures were captured using both 100 × and 400 × objectives. (E,G) Statistical analysis were performed to demonstrate whether the expression levels of IL-18 and IL-1β in normal brain tissues and gliomas are correlated with histological grades. (H,I) Tissue homogenates of normal brain tissue and gliomas was collected and assayed for IL-1β and IL-18 using human ELISA kits. (J,K ) mRNA levels of IL-1β and IL-18 between normal brain tissues and gliomas were compared by qPCR. Unpaired student _t_-test was applied for the statistical analysis, p < 0.05 was considered as statistically significant.
Figure 3
Caspase1 inactivation significantly decreased the protein expression of IL-18 and IL-1β and led to U87 cell death. (A) U87 cells were either sham-treated or with Ac-YYAD-CMK at 50 and 100 μM for 24 h. The cell lysates were collected and processed for western blotting of caspase-1, and β-actin was used as a loading control. (B) Protein levels of cleaved caspase-1 was quantified by densitometric analysis using NIH ImageJ as described. (C) mRNA was extracted from U87 cells treated with either 50 or 100 μM Ac-YYAD-CMK. mRNA levels of caspase-1 was analyzed by qPCR as described. (D,E) Cell supernatants of U87 cells treated with either 50 or 100 μM Ac-YYAD-CMK was collected and assayed for IL-1β and IL-18 using human ELISA kits. (F) Cell viability was determined by the MTT assay at multiple time points after Ac-YYAD-CMK treatment. (G,H) Cell migration was examined using the wound scratch assay in U87 cells treated with caspase-1 inhibitor at different dosages as indicated for 48 h. Cell migration was quantified as the reduction of the wound square as described in the section Materials and Methods.
Figure 4
Inhibitory effects of berberine on protein expression of caspase-1, IL-18 and IL-1β. (A) U87 cells were either sham-treated or with berberine at 50 and 100 μM for 24 h. The cell lysates were collected and processed for western blotting of caspase-1, and β-actin was used as a loading control. (B) Protein levels of cleaved caspase-1 was quantified by densitometric analysis using NIH ImageJ as described. (C) mRNA was extracted from U87 cells treated with either 50 or 100 μM berberine. mRNA levels of caspase-1 was analyzed by qPCR as described. (D,E) Cell supernatants of U87 cells treated with either 50 or 100 μM berberine was collected and assayed for IL-1β and IL-18 using human ELISA kits. (F,G) mRNA was extracted from U87 cells treated with either 50 or 100 μM berberine. mRNA levels of IL-1β and IL-18 were analyzed by qPCR as described.
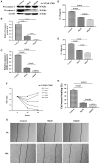
Figure 5
The effects of berberine on cell viability and cell migration. (A–C) Cell viability was determined by the MTT assay at multiple time points after berberine treatment (A) U87, (B) U251, and (C) Oligodendrocyte. (D,F) Cell migration was examined using the wound scratch assay in U87 and U251 cells treated with berberine at different dosage as indicated for 48 h. (E,G) Cell migration was quantified as the reduction of the wound square as described in the section Materials and Methods.
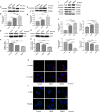
Figure 6
The effects of berberine treatment on EMT process. U87 and U251 cells were either sham-treated or with berberine at 50 and 100 μM for 48 h. The cell lysates were collected and processed for western blotting of β-catenin, α-catenin, vimentin and α-SMA, and β-actin was used as a loading control (A,C,E,G,I). Protein levels of β-catenin, α-catenin, vimentin, and α-SMA were quantified by densitometric analysis using NIH ImageJ as described (B,D,F,H,J). U87 cells were treated with berberine as above and then immunocytochemical staining was conducted using anti-β-catenin (red), anti-α-catenin (red), anti-vimentin (green), and anti-α-SMA antibodies (green). Nuclei were counterstained using DAPI (blue). Scale bars are 10 μM (K,L).
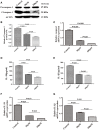
Figure 7
Caspase-1 inactivation prevents EMT in U87 and U251 cells. U87 and U251 cells were either sham-treated or with Ac-YYAD-CMK at 50 and 100 μM for 48 h. The cell lysates were collected and processed for western blotting of β-catenin, α-catenin, vimentin and α-SMA, and β-actin was used as a loading control (A,C). Protein levels of β-catenin, α-catenin, vimentin, and α-SMA were quantified by densitometric analysis using NIH ImageJ as described (B,D).
Figure 8
Berberine inhibits caspase-1 activation via the ERK1/2 signaling pathway. (A,C) The cell lysates were collected and processed for western blotting of p-ERK and caspase-1, and β-actin was used as a loading control. (B,D) Protein levels of p-ERK and caspase-1 were quantified by densitometric analysis using NIH ImageJ as described.
Similar articles
- Berberine affects osteosarcoma via downregulating the caspase-1/IL-1β signaling axis.
Jin H, Jin X, Cao B, Wang W. Jin H, et al. Oncol Rep. 2017 Feb;37(2):729-736. doi: 10.3892/or.2016.5327. Epub 2016 Dec 16. Oncol Rep. 2017. PMID: 28000894 Free PMC article. - Oncosis-like cell death is induced by berberine through ERK1/2-mediated impairment of mitochondrial aerobic respiration in gliomas.
Sun Y, Yu J, Liu X, Zhang C, Cao J, Li G, Liu X, Chen Y, Huang H. Sun Y, et al. Biomed Pharmacother. 2018 Jun;102:699-710. doi: 10.1016/j.biopha.2018.03.132. Epub 2018 Apr 5. Biomed Pharmacother. 2018. PMID: 29604589 - Nano strategies for berberine delivery, a natural alkaloid of Berberis.
Mirhadi E, Rezaee M, Malaekeh-Nikouei B. Mirhadi E, et al. Biomed Pharmacother. 2018 Aug;104:465-473. doi: 10.1016/j.biopha.2018.05.067. Epub 2018 May 25. Biomed Pharmacother. 2018. PMID: 29793179 Review. - Berberine and neurodegeneration: A review of literature.
Ahmed T, Gilani AU, Abdollahi M, Daglia M, Nabavi SF, Nabavi SM. Ahmed T, et al. Pharmacol Rep. 2015 Oct;67(5):970-9. doi: 10.1016/j.pharep.2015.03.002. Epub 2015 Mar 18. Pharmacol Rep. 2015. PMID: 26398393 Review.
Cited by
- The effect of berberine chloride and/or its combination with vancomycin on the growth, biofilm formation, and motility of Clostridioides difficile.
Wultańska D, Piotrowski M, Pituch H. Wultańska D, et al. Eur J Clin Microbiol Infect Dis. 2020 Jul;39(7):1391-1399. doi: 10.1007/s10096-020-03857-0. Epub 2020 Mar 5. Eur J Clin Microbiol Infect Dis. 2020. PMID: 32140903 Free PMC article. - A Novel Prognostic Pyroptosis-Related Gene Signature Correlates to Oxidative Stress and Immune-Related Features in Gliomas.
Zeng S, Li W, Ouyang H, Xie Y, Feng X, Huang L. Zeng S, et al. Oxid Med Cell Longev. 2023 Jan 31;2023:4256116. doi: 10.1155/2023/4256116. eCollection 2023. Oxid Med Cell Longev. 2023. PMID: 36778205 Free PMC article. - Systematic transcriptome profiling of pyroptosis related signature for predicting prognosis and immune landscape in lower grade glioma.
Yu H, Gong M, Qi J, Zhao C, Niu W, Sun S, Li S, Hong B, Qian J, Wang H, Chen X, Fang Z. Yu H, et al. BMC Cancer. 2022 Aug 13;22(1):885. doi: 10.1186/s12885-022-09982-7. BMC Cancer. 2022. PMID: 35964070 Free PMC article. - Potential of Natural Products in the Treatment of Glioma: Focus on Molecular Mechanisms.
Sheida A, Farshadi M, Mirzaei A, Najjar Khalilabad S, Zarepour F, Taghavi SP, Hosseini Khabr MS, Ravaei F, Rafiei S, Mosadeghi K, Yazdani MS, Fakhraie A, Ghattan A, Zamani Fard MM, Shahyan M, Rafiei M, Rahimian N, Talaei Zavareh SA, Mirzaei H. Sheida A, et al. Cell Biochem Biophys. 2024 Dec;82(4):3157-3208. doi: 10.1007/s12013-024-01447-x. Epub 2024 Aug 16. Cell Biochem Biophys. 2024. PMID: 39150676 Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous