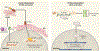The Cytosolic DNA-Sensing cGAS-STING Pathway in Cancer - PubMed (original) (raw)
Review
The Cytosolic DNA-Sensing cGAS-STING Pathway in Cancer
John Kwon et al. Cancer Discov. 2020 Jan.
Abstract
The recognition of DNA as an immune-stimulatory molecule is an evolutionarily conserved mechanism to initiate rapid innate immune responses against microbial pathogens. The cGAS-STING pathway was discovered as an important DNA-sensing machinery in innate immunity and viral defense. Recent advances have now expanded the roles of cGAS-STING to cancer. Highly aggressive, unstable tumors have evolved to co-opt this program to drive tumorigenic behaviors. In this review, we discuss the link between the cGAS-STING DNA-sensing pathway and antitumor immunity as well as cancer progression, genomic instability, the tumor microenvironment, and pharmacologic strategies for cancer therapy. SIGNIFICANCE: The cGAS-STING pathway is an evolutionarily conserved defense mechanism against viral infections. Given its role in activating immune surveillance, it has been assumed that this pathway primarily functions as a tumor suppressor. Yet, mounting evidence now suggests that depending on the context, cGAS-STING signaling can also have tumor and metastasis-promoting functions, and its chronic activation can paradoxically induce an immune-suppressive tumor microenvironment.
©2019 American Association for Cancer Research.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
SFB holds a patent related to some of the work described targeting CIN and the cGAS-STING pathway in advanced cancer. He owns equity in, receives compensation from, and serves as a consultant and the Scientific Advisory Board and Board of Directors of Volastra Pharmaceuticals Inc. He has also consulted for Sanofi. JK declares no conflicts of interest.
Figures
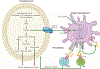
Figure 1.. cGAS-STING signaling in immunity.
cGAS is an innate immune sensor that recognizes a diverse array of cytosolic dsDNA, which includes DNA with viral, apoptotic, exosomal, mitochondrial, micronuclei, and retroelement origins. cGAS oligomerizes with dsDNA in a 2:2 complex. The interaction of cGAS with DNA induces the formation of liquid droplets through phase transition, in which cGAS exerts its catalytic role to generate the second messenger 2’,3’-cGAMP. The presence of cGAMP stimulates STING at the ER, which undergoes higher-ordered tetramerization. STING translocates from the ER to Golgi compartments and is palmitoylated. STING serves as a signaling platform for TBK1 and IKK. TBK1 phosphorylates STING, which in turn recruits IRF3 for TBK1-mediated phosphorylation. Activated IRF3 dimerizes and translocates to the nucleus. IKK-mediated phosphorylation of the inhibitory IκB protein licenses nuclear entry of p50-RelA. Together with IRF3 in the nucleus, p50-RelA dimers stimulate the transcriptional expression of interferon and other immune-stimulatory genes. Artwork by Terry Helms, Graphics Department, MSKCC.
Figure 2.. Independent functions cGAS and STING.
cGAS and STING possess alternative functions that diverge from the uniaxial and canonical cGAS-cGAMP-STING pathway, exerting effects that are independent from each other. Nuclear translocation of cytosolic cGAS has been shown to drive tumorigenesis through the blockade of HR repair in a STING-independent manner. Breakdown of the nuclear membrane in mitosis has been suggested to allow nuclear entry of cytosolic cGAS, which remains catalytically active and binds to centromeric satellites and LINE DNA repeats. On the other hand, STING can also be activated in a cGAS-independent manner. Etoposide-induced DNA damage recruits the DNA-damage response factors PARP-1 and ATM, which in turn activates STING to drive canonical NF-κB and IRF3-mediated interferon signaling. Artwork by Terry Helms, Graphics Department, MSKCC.
Figure 3.. Tumor Suppressive Roles of cGAS-STING.
In early preneoplastic cells, the cGAS-STING pathway exerts its function as a tumor suppressor against oncogenic effects induced by DNA damage. Several sources of DNA damage, such as oxidative stress, radiation, low chromosome instability, hyper-activation of oncogene signaling, and chemotherapies, generates cytosolic DNA, which is sensed by cGAS within tumors. cGAS, in turn stimulates STING to upregulate the expression of type 1 IFN, ISG, and SASP genes. Through an unknown mechanism, STING-mediated autophagy may cooperate with IRF3 and canonical NF-κB signaling to inhibit or delay cancer progression. In addition, the cGAS-STING pathway allows crosstalk between tumors and neighboring immune cells to regulate antitumor immunity. Tumor-derived cGAMP and tumor DNA licenses antigen presenting cells, such as dendritic cells, to activate cGAS-STING signaling, triggering immune cells to mediate tumor clearance. Artwork by Terry Helms, Graphics Department, MSKCC.
Figure 4.. Tumor Promoting Roles of cGAS-STING.
In metastatic tumor cells, the cGAS-STING pathway exerts its role in promoting tumorigenesis. Tumors with high chromosome instability generate micronuclei, which ruptures and releases DNA to the cytosol. STING serves as a signaling platform for a diverse array of tumorigenic programs. Chronic activation of STING signaling leads to suppression of type 1 IFN production and concurrent upregulation of noncanonical NF-κB signaling, pro-survival programs and metastasis. The release of immune-stimulating molecules, such as indoleamine 2,3-dioxygenase (IDO) and anti-inflammatory cytokines, from tumors allows the formation of an immunosuppressive microenvironment. STING may have additional roles in promoting immune evasion and metastasis through regulation of PD-L1 expression and induction of autophagy. Apart from cancer cell-autonomous roles of cGAS-STING, tumors can directly communicate with their external environment through the transfer of cGAMP to neighboring cells through gap junctions, which accelerates neoplastic progression. Artwork by Terry Helms, Graphics Department, MSKC.
Similar articles
- The cGAS-STING Pathway: Novel Perspectives in Liver Diseases.
Xu D, Tian Y, Xia Q, Ke B. Xu D, et al. Front Immunol. 2021 Apr 29;12:682736. doi: 10.3389/fimmu.2021.682736. eCollection 2021. Front Immunol. 2021. PMID: 33995425 Free PMC article. Review. - Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing.
Chen Q, Sun L, Chen ZJ. Chen Q, et al. Nat Immunol. 2016 Sep 20;17(10):1142-9. doi: 10.1038/ni.3558. Nat Immunol. 2016. PMID: 27648547 Review. - The pleiotropic roles of cGAS-STING signaling in the tumor microenvironment.
Li J, Bakhoum SF. Li J, et al. J Mol Cell Biol. 2022 Aug 5;14(4):mjac019. doi: 10.1093/jmcb/mjac019. J Mol Cell Biol. 2022. PMID: 35325182 Free PMC article. Review. - cGAS-STING Activation in the Tumor Microenvironment and Its Role in Cancer Immunity.
Pépin G, Gantier MP. Pépin G, et al. Adv Exp Med Biol. 2017;1024:175-194. doi: 10.1007/978-981-10-5987-2_8. Adv Exp Med Biol. 2017. PMID: 28921470 Review. - Comprehensive elaboration of the cGAS-STING signaling axis in cancer development and immunotherapy.
Zheng J, Mo J, Zhu T, Zhuo W, Yi Y, Hu S, Yin J, Zhang W, Zhou H, Liu Z. Zheng J, et al. Mol Cancer. 2020 Aug 27;19(1):133. doi: 10.1186/s12943-020-01250-1. Mol Cancer. 2020. PMID: 32854711 Free PMC article. Review.
Cited by
- An insight into the role of innate immune cells in breast tumor microenvironment.
Garg S, Rai G, Singh S, Gauba P, Ali J, Dang S. Garg S, et al. Breast Cancer. 2025 Jan;32(1):79-100. doi: 10.1007/s12282-024-01645-8. Epub 2024 Oct 26. Breast Cancer. 2025. PMID: 39460874 Review. - High prevalence of chromosome 17 in breast cancer micronuclei: a means to get rid of tumor suppressors?
Kumari L, Sreedharanunni S, Dahiya D, Dey P, Bhatia A. Kumari L, et al. Hum Cell. 2024 Oct 22;38(1):5. doi: 10.1007/s13577-024-01143-1. Hum Cell. 2024. PMID: 39438374 - C5aR2 Regulates STING-Mediated Interferon Beta Production in Human Macrophages.
Wright O, Harris A, Nguyen VD, Zhou Y, Durand M, Jayyaratnam A, Gormley D, O'Neill LAJ, Triantafilou K, Nichols EM, Booty LM. Wright O, et al. Cells. 2023 Nov 25;12(23):2707. doi: 10.3390/cells12232707. Cells. 2023. PMID: 38067135 Free PMC article. - Impact of Genomic Mutation on Melanoma Immune Microenvironment and IFN-1 Pathway-Driven Therapeutic Responses.
Mentucci FM, Romero Nuñez EA, Ercole A, Silvetti V, Dal Col J, Lamberti MJ. Mentucci FM, et al. Cancers (Basel). 2024 Jul 17;16(14):2568. doi: 10.3390/cancers16142568. Cancers (Basel). 2024. PMID: 39061208 Free PMC article. - STING pathway contributes to the prognosis of hepatocellular carcinoma and identification of prognostic gene signatures correlated to tumor microenvironment.
Pu Z, Liu J, Liu Z, Peng F, Zhu Y, Wang X, He J, Yi P, Hu X, Fan X, Chen J. Pu Z, et al. Cancer Cell Int. 2022 Oct 12;22(1):314. doi: 10.1186/s12935-022-02734-4. Cancer Cell Int. 2022. PMID: 36224658 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials