Effect of Dapagliflozin on Outpatient Worsening of Patients With Heart Failure and Reduced Ejection Fraction: A Prespecified Analysis of DAPA-HF - PubMed (original) (raw)
Randomized Controlled Trial
. 2020 Oct 27;142(17):1623-1632.
doi: 10.1161/CIRCULATIONAHA.120.047480. Epub 2020 Sep 4.
Pardeep S Jhund # 1, Inder Anand 2, Olof Bengtsson 3, Michael Böhm 4, Rudolf A de Boer 5, David L DeMets 6, Akshay S Desai 7, Jaroslaw Drozdz 8, Jonathan Howlett 9, Silvio E Inzucchi 10, Per Johanson 3, Tzvetana Katova 11, Lars Køber 12, Mikhail N Kosiborod 13 14, Anna Maria Langkilde 3, Daniel Lindholm 3, Felipe A Martinez 15, Béla Merkely 16, Jose C Nicolau 17, Eileen O'Meara 18, Piotr Ponikowski 19, Marc S Sabatine 20, Mikaela Sjöstrand 3, Scott D Solomon 7, Sergey Tereshchenko 21, Subodh Verma 22, John J V McMurray 1
Affiliations
- PMID: 32883108
- PMCID: PMC7580857
- DOI: 10.1161/CIRCULATIONAHA.120.047480
Randomized Controlled Trial
Effect of Dapagliflozin on Outpatient Worsening of Patients With Heart Failure and Reduced Ejection Fraction: A Prespecified Analysis of DAPA-HF
Kieran F Docherty et al. Circulation. 2020.
Abstract
Background: In the DAPA-HF trial (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure), dapagliflozin, added to guideline-recommended therapies, reduced the risk of mortality and heart failure (HF) hospitalization. We examined the frequency and significance of episodes of outpatient HF worsening, requiring the augmentation of oral therapy, and the effects of dapagliflozin on these additional events.
Methods: Patients in New York Heart Association functional class II to IV, with a left ventricular ejection fraction ≤40% and elevation of NT-proBNP (N-terminal pro-B-type natriuretic peptide), were eligible. The primary outcome was the composite of an episode of worsening HF (HF hospitalization or an urgent HF visit requiring intravenous therapy) or cardiovascular death, whichever occurred first. An additional prespecified exploratory outcome was the primary outcome plus worsening HF symptoms/signs leading to the initiation of new, or the augmentation of existing, oral treatment.
Results: Overall, 36% more patients experienced the expanded, in comparison with the primary, composite outcome. In the placebo group, 684 of 2371 (28.8%) patients and, in the dapagliflozin group, 527 of 2373 (22.2%) participants experienced the expanded outcome (hazard ratio, 0.73 [95% CI, 0.65-0.82]; P<0.0001). Each component of the composite was reduced significantly by dapagliflozin. Over the median follow-up of 18.2 months, the number of patients needed to treat with dapagliflozin to prevent 1 experiencing an episode of fatal or nonfatal worsening was 16. Among the 4744 randomly assigned patients, the first episode of worsening was outpatient augmentation of treatment in 407 participants (8.6%), an urgent HF visit with intravenous therapy in 20 (0.4%), HF hospitalization in 489 (10.3%), and cardiovascular death in 295 (6.2%). The adjusted risk of death from any cause (in comparison with no event) after an outpatient worsening was hazard ratio, 2.67 (95% CI, 2.03-3.52); after an urgent HF visit, the adjusted risk of death was hazard ratio, 3.00 (95% CI, 1.39-6.48); and after a HF hospitalization, the adjusted risk of death was hazard ratio, 6.21 (95% CI, 5.07-7.62).
Conclusion: In DAPA-HF, outpatient episodes of HF worsening were common, were of prognostic importance, and were reduced by dapagliflozin. Registration: URL: https://www.clinicaltrials.gov; Unique Identifier: NCT03036124.
Keywords: heart failure; hospitalization; sodium-glucose transporter 2 inhibitors; therapy; treatment outcome.
Figures
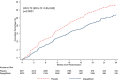
Figure 1.
Kaplan-Meier curves for the expanded composite outcome of time to first cardiovascular death, hospitalization for heart failure, urgent heart failure visit, or outpatient intensification of heart failure therapy, according to treatment group. Hazard ratios (HR) and 95% CIs were estimated with the use of Cox regression models, stratified according to diabetes status, with a history of hospitalization for heart failure and treatment-group assignment as explanatory variables.
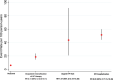
Figure 2.
Effect of dapagliflozin versus placebo on manifestations of worsening heart failure. Hazard ratios and 95% CIs were estimated with the use of Cox regression models, stratified according to diabetes status, with a history of hospitalization for heart failure and treatment-group assignment as explanatory variables. CV indicates cardiovascular; and HF, heart failure.
Figure 3.
Rate and risk of all-cause mortality after a first nonfatal heart failure worsening event. The risk of each death from any cause relative to patient not experiencing an event after a first nonfatal heart failure worsening event is calculated by using Cox regression models with the event entered in the model as a time-updated covariate. HF indicates heart failure; and HR, hazard ratio.
Similar articles
- Efficacy and Safety of Dapagliflozin in Men and Women With Heart Failure With Reduced Ejection Fraction: A Prespecified Analysis of the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure Trial.
Butt JH, Docherty KF, Petrie MC, Schou M, Kosiborod MN, O'Meara E, Katova T, Ljungman CEA, Diez M, Ogunniyi MO, Langkilde AM, Sjöstrand M, Lindholm D, Bengtsson O, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, Jhund PS, McMurray JJV, Køber L. Butt JH, et al. JAMA Cardiol. 2021 Jun 1;6(6):678-689. doi: 10.1001/jamacardio.2021.0379. JAMA Cardiol. 2021. PMID: 33787831 Free PMC article. Clinical Trial. - Efficacy and Safety of Dapagliflozin in Heart Failure With Reduced Ejection Fraction According to N-Terminal Pro-B-Type Natriuretic Peptide: Insights From the DAPA-HF Trial.
Butt JH, Adamson C, Docherty KF, de Boer RA, Petrie MC, Inzucchi SE, Kosiborod MN, Maria Langkilde A, Lindholm D, Martinez FA, Bengtsson O, Schou M, O'Meara E, Ponikowski P, Sabatine MS, Sjöstrand M, Solomon SD, Jhund PS, McMurray JJV, Køber L. Butt JH, et al. Circ Heart Fail. 2021 Dec;14(12):e008837. doi: 10.1161/CIRCHEARTFAILURE.121.008837. Epub 2021 Nov 22. Circ Heart Fail. 2021. PMID: 34802253 - Efficacy and Safety of Dapagliflozin in Heart Failure With Reduced Ejection Fraction According to Age: Insights From DAPA-HF.
Martinez FA, Serenelli M, Nicolau JC, Petrie MC, Chiang CE, Tereshchenko S, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Ponikowski P, Sabatine MS, DeMets DL, Dutkiewicz-Piasecka M, Bengtsson O, Sjöstrand M, Langkilde AM, Jhund PS, McMurray JJV. Martinez FA, et al. Circulation. 2020 Jan 14;141(2):100-111. doi: 10.1161/CIRCULATIONAHA.119.044133. Epub 2019 Nov 17. Circulation. 2020. PMID: 31736328 Clinical Trial. - Dapagliflozin: A Review in Symptomatic Heart Failure with Reduced Ejection Fraction.
Blair HA. Blair HA. Am J Cardiovasc Drugs. 2021 Nov;21(6):701-710. doi: 10.1007/s40256-021-00503-8. Epub 2021 Oct 15. Am J Cardiovasc Drugs. 2021. PMID: 34651263 Free PMC article. Review. - Empagliflozin and other SGLT2 inhibitors in patients with heart failure and preserved ejection fraction: a systematic review and meta-analysis.
Hamid AK, Tayem AA, Al-Aish ST, Al Sakini AS, Hadi DD, Al-Aish RT. Hamid AK, et al. Ther Adv Cardiovasc Dis. 2024 Jan-Dec;18:17539447241289067. doi: 10.1177/17539447241289067. Ther Adv Cardiovasc Dis. 2024. PMID: 39400108 Free PMC article. Review.
Cited by
- Patient Selection and End Point Definitions for Decongestion Studies in Acute Decompensated Heart Failure: Part 1.
Georges G, Fudim M, Burkhoff D, Leon MB, Généreux P. Georges G, et al. J Soc Cardiovasc Angiogr Interv. 2023 Aug 8;2(6Part B):101060. doi: 10.1016/j.jscai.2023.101060. eCollection 2023 Nov-Dec. J Soc Cardiovasc Angiogr Interv. 2023. PMID: 39131061 Free PMC article. Review. - Semaglutide and diuretic use in obesity-related heart failure with preserved ejection fraction: a pooled analysis of the STEP-HFpEF and STEP-HFpEF-DM trials.
Shah SJ, Sharma K, Borlaug BA, Butler J, Davies M, Kitzman DW, Petrie MC, Verma S, Patel S, Chinnakondepalli KM, Einfeldt MN, Jensen TJ, Rasmussen S, Asleh R, Ben-Gal T, Kosiborod MN. Shah SJ, et al. Eur Heart J. 2024 Sep 14;45(35):3254-3269. doi: 10.1093/eurheartj/ehae322. Eur Heart J. 2024. PMID: 38739118 Free PMC article. Clinical Trial. - Effect of Empagliflozin on Heart Failure Outcomes After Acute Myocardial Infarction: Insights From the EMPACT-MI Trial.
Hernandez AF, Udell JA, Jones WS, Anker SD, Petrie MC, Harrington J, Mattheus M, Seide S, Zwiener I, Amir O, Bahit MC, Bauersachs J, Bayes-Genis A, Chen Y, Chopra VK, A Figtree G, Ge J, G Goodman S, Gotcheva N, Goto S, Gasior T, Jamal W, Januzzi JL, Jeong MH, Lopatin Y, Lopes RD, Merkely B, Parikh PB, Parkhomenko A, Ponikowski P, Rossello X, Schou M, Simic D, Steg PG, Szachniewicz J, van der Meer P, Vinereanu D, Zieroth S, Brueckmann M, Sumin M, Bhatt DL, Butler J. Hernandez AF, et al. Circulation. 2024 May 21;149(21):1627-1638. doi: 10.1161/CIRCULATIONAHA.124.069217. Epub 2024 Apr 6. Circulation. 2024. PMID: 38581389 Free PMC article. Clinical Trial. - The Safety and Efficacy of Sodium-Glucose Cotransporter-2 Inhibitors for Patients with Sarcopenia or Frailty: Double Edged Sword?
Naito A, Nagatomo Y, Kawai A, Yukino-Iwashita M, Nakazawa R, Taruoka A, Takefuji A, Yasuda R, Toya T, Ikegami Y, Masaki N, Ido Y, Adachi T. Naito A, et al. J Pers Med. 2024 Jan 26;14(2):141. doi: 10.3390/jpm14020141. J Pers Med. 2024. PMID: 38392575 Free PMC article. Review. - Current Role of SLGT2 Inhibitors in the Management of the Whole Spectrum of Heart Failure: Focus on Dapagliflozin.
Escobar C, Pascual-Figal D, Manzano L, Nuñez J, Camafort M. Escobar C, et al. J Clin Med. 2023 Oct 27;12(21):6798. doi: 10.3390/jcm12216798. J Clin Med. 2023. PMID: 37959263 Free PMC article. Review.
References
- Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L; SHIFT Investigators Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61198-1 - PubMed
- Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B; EMPHASIS-HF Study Group Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492 - PubMed
- McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, et al. ; PARADIGM-HF Investigators and Committees Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077 - PubMed
- McMurray JJ, Krum H, Abraham WT, Dickstein K, Køber LV, Desai AS, Solomon SD, Greenlaw N, Ali MA, Chiang Y, et al. ; ATMOSPHERE Committees Investigators Aliskiren, enalapril, or aliskiren and enalapril in heart failure. N Engl J Med. 2016;374:1521–1532. doi: 10.1056/NEJMoa1514859 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous