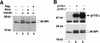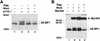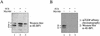4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway - PubMed (original) (raw)
4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway
A C Gingras et al. Genes Dev. 1998.
Abstract
Growth factors and hormones activate protein translation by phosphorylation and inactivation of the translational repressors, the eIF4E-binding proteins (4E-BPs), through a wortmannin- and rapamycin-sensitive signaling pathway. The mechanism by which signals emanating from extracellular signals lead to phosphorylation of 4E-BPs is not well understood. Here we demonstrate that the activity of the serine/threonine kinase Akt/PKB is required in a signaling cascade that leads to phosphorylation and inactivation of 4E-BP1. PI 3-kinase elicits the phosphorylation of 4E-BP1 in a wortmannin- and rapamycin-sensitive manner, whereas activated Akt-mediated phosphorylation of 4E-BP1 is wortmannin resistant but rapamycin sensitive. A dominant negative mutant of Akt blocks insulin-mediated phosphorylation of 4E-BP1, indicating that Akt is required for the in vivo phosphorylation of 4E-BP1. Importantly, an activated Akt induces phosphorylation of 4E-BP1 on the same sites that are phosphorylated upon serum stimulation. Similar to what has been observed with serum and growth factors, phosphorylation of 4E-BP1 by Akt inhibits the interaction between 4E-BP1 and eIF-4E. Furthermore, phosphorylation of 4E-BP1 by Akt requires the activity of FRAP/mTOR. FRAP/mTOR may lie downstream of Akt in this signaling cascade. These results demonstrate that the PI 3-kinase-Akt signaling pathway, in concert with FRAP/mTOR, induces the phosphorylation of 4E-BP1.
Figures
Figure 1
PI 3-kinase and Akt elicit phosphorylation of 4E-BP1. (A) Insulin-mediated phosphorylation of 4E-BP1 is both rapamycin and wortmannin sensitive. Human embryonic kidney (HEK) 293 cells were transfected transiently with a hemaglutinin (HA) epitope-tagged 4E-BP1 expression vector. After transfection, cells were deprived of serum for 36 hr, and either mock treated (lane 1) or stimulated with insulin (1 μg/ml) for 30 min (lanes 2–4) in the presence of either wortmannin, [200 n
m
(Wort.)] (lane 3) or rapamycin [20 ng/ml (Rap.)] (lane 4). Cell extracts were prepared as described in Materials and Methods and HA–4E-BP1 was detected by immunoblot analysis with an anti-HA antibody (12CA5). Molecular size markers (in kD) are indicated. Arrows indicate the different phosphorylated isoforms of HA–4E-BP1. (B) The catalytic subunit of PI 3-kinase p110α elicits phosphorylation of HA–4E-BP1. HEK 293 cells were cotransfected with HA–4E-BP1 expression vector along with one of the following: control vector (lane 1), p110α expression vector (lane 2), or p110αcaax (p110α*) expression vector (lane 3). After transfection, cells were deprived of serum for 36 hr. HA–4E-BP1 was detected as described in A. Small arrows indicate the different phosphorylation forms of 4E-BP1. p110α and p110αcaax were detected as described in Materials and Methods. (C) Akt elicits phosphorylation of HA–4E-BP1. HEK 293 cells were mock transfected (lane 1) or cotransfected with HA–4E-BP1 expression vector and one of the following: control vector (lane 2), HA–c-Akt expression vector (lane 3), or HA–MyrAkt expression vector (lane 4). Cells were deprived of serum for 36 hr. HA–4E-BP1 was detected as described above. Small arrows indicate the different phosphorylation forms of 4E-BP1. (D) A kinase-deficient mutant of Akt inhibits phosphorylation of 4E-BP1 by insulin. HEK 293 cells were cotransfected with a HA–4E-BPI expression vector (100 ng) and the following: control vector (lanes 1,2) or HA–AktK179M expression vector [Akt(kin−)] (lanes 3,4). After transfection, cells were serum-deprived for 36 hr and then stimulated with 100 ng/ml of insulin for 45 min (lanes 2,4). HA–4E-BP1 was detected as described above. HA–AktK179M was detected on the same immunoblot. Small arrows indicate the different phosphorylated forms of 4E-BP1. The results shown are representative of three independent experiments.
Figure 1
PI 3-kinase and Akt elicit phosphorylation of 4E-BP1. (A) Insulin-mediated phosphorylation of 4E-BP1 is both rapamycin and wortmannin sensitive. Human embryonic kidney (HEK) 293 cells were transfected transiently with a hemaglutinin (HA) epitope-tagged 4E-BP1 expression vector. After transfection, cells were deprived of serum for 36 hr, and either mock treated (lane 1) or stimulated with insulin (1 μg/ml) for 30 min (lanes 2–4) in the presence of either wortmannin, [200 n
m
(Wort.)] (lane 3) or rapamycin [20 ng/ml (Rap.)] (lane 4). Cell extracts were prepared as described in Materials and Methods and HA–4E-BP1 was detected by immunoblot analysis with an anti-HA antibody (12CA5). Molecular size markers (in kD) are indicated. Arrows indicate the different phosphorylated isoforms of HA–4E-BP1. (B) The catalytic subunit of PI 3-kinase p110α elicits phosphorylation of HA–4E-BP1. HEK 293 cells were cotransfected with HA–4E-BP1 expression vector along with one of the following: control vector (lane 1), p110α expression vector (lane 2), or p110αcaax (p110α*) expression vector (lane 3). After transfection, cells were deprived of serum for 36 hr. HA–4E-BP1 was detected as described in A. Small arrows indicate the different phosphorylation forms of 4E-BP1. p110α and p110αcaax were detected as described in Materials and Methods. (C) Akt elicits phosphorylation of HA–4E-BP1. HEK 293 cells were mock transfected (lane 1) or cotransfected with HA–4E-BP1 expression vector and one of the following: control vector (lane 2), HA–c-Akt expression vector (lane 3), or HA–MyrAkt expression vector (lane 4). Cells were deprived of serum for 36 hr. HA–4E-BP1 was detected as described above. Small arrows indicate the different phosphorylation forms of 4E-BP1. (D) A kinase-deficient mutant of Akt inhibits phosphorylation of 4E-BP1 by insulin. HEK 293 cells were cotransfected with a HA–4E-BPI expression vector (100 ng) and the following: control vector (lanes 1,2) or HA–AktK179M expression vector [Akt(kin−)] (lanes 3,4). After transfection, cells were serum-deprived for 36 hr and then stimulated with 100 ng/ml of insulin for 45 min (lanes 2,4). HA–4E-BP1 was detected as described above. HA–AktK179M was detected on the same immunoblot. Small arrows indicate the different phosphorylated forms of 4E-BP1. The results shown are representative of three independent experiments.
Figure 2
Effect of wortmannin and rapamycin on 4E-BP1 phosphorylation by Akt and PI 3-kinase. (A) Phosphorylation of 4E-BP1 by p110αcaax is both wortmannin and rapamycin sensitive. HEK 293 cells were cotransfected with HA–4E-BP1 expression vector and one of the following: control vector (lane 1) or p110αcaax (p110α*) expression vector (lanes 2–4). After transfection, cells were deprived of serum for 36 hr and treated with either wortmannin [200 n
m
(Wort.); lane _3_] or rapamycin [20 ng/ml (Rap.); lane _4_]. Cell extracts were prepared and HA–4E–BP1 was detected as described in Fig. 1. Arrows indicate different phosphorylation isoforms of 4E-BP1. (B) Phosphorylation of 4E-BP1 induced by an activated Akt is wortmannin resistant but rapamycin sensitive. HEK 293 cells were cotransfected with HA–4E-BP1 expression vector and with control vector (lane 1), or with HA–MyrAkt expression vector (lanes 2–4). After transfection, cells were deprived of serum for 36 hr. Cells were treated and 4E-BP1 was detected as described in A. HA–MyrAkt was detected on the same immunoblot. Small arrows indicate different phosphorylation forms of 4E-BP1. The figure is representative of two independent experiments.
Figure 3
4E-BP1 and 4E-BP2 32P incorporation is increased in 293 MyrAkt cells and is resistant to wortmannin treatment. Cells were labeled with [32P]orthophosphate as described in Materials and Methods and 4E-BP1 (A) and 4E-BP2 (B) were immunoprecipitated successively with polyclonal antibodies, separated by SDS-PAGE, transferred to Immobilon-PSQ and subjected to autoradiography. Different phosphorylated isoforms are indicated for 4E-BP1.
Figure 3
4E-BP1 and 4E-BP2 32P incorporation is increased in 293 MyrAkt cells and is resistant to wortmannin treatment. Cells were labeled with [32P]orthophosphate as described in Materials and Methods and 4E-BP1 (A) and 4E-BP2 (B) were immunoprecipitated successively with polyclonal antibodies, separated by SDS-PAGE, transferred to Immobilon-PSQ and subjected to autoradiography. Different phosphorylated isoforms are indicated for 4E-BP1.
Figure 4
The phosphopeptide map of 4E-BP1 in 293 MyrAkt cells is identical to that of serum-stimulated 293 cells. 32P-Labeled 4E-BP1 (Fig. 3) was excised from an Immobilon membrane, digested with trypsin–chymotrypsin, and analyzed by two-dimensional phosphopeptide mapping, as described in Materials and Methods. HEK 293 cells (A–D) and HEK 293/ MyrAkt cells (E–G) were deprived of serum for 36 hr. Cells were labeled with 32P as described in Materials and Methods. (A,E) Untreated cells (B–D,F,G) were treated as follows: with 15% FCS for 30 min (B); pretreated with rapamycin (20 ng/ml) for 20 min before addition of FCS (C); pretreated with wortmannin (100 n
m
) for 20 min before addition of FCS (D); with rapamycin (20 ng/ml) for 20 min (F); with wortmannin (100 n
m
) for 20 min (G).
Figure 5
Phosphorylation of 4E-BP1 by Akt inhibits interaction with eIF4E. Rat1a and Rat1a/MyrAkt cells were incubated in 0.5% FCS overnight. Rat1a cells were then treated with 20% FCS for 40 min. Cells were lysed by freeze–thaw cycles and extracts were either heat treated (total extract; 100 μg) or incubated (750 μg) with m7GDP–agarose resin, as described in Materials and Methods. Samples were separated by SDS-PAGE and 4E-BP1 protein was analyzed by Western blotting. (A) Total extract (100 μg). (B) Material bound to the m7GDP–agarose resin. Positions of the 4E-BP1 isoforms are indicated.
Figure 6
Akt does not phosphorylate 4E-BP1 in vitro. HEK 293 cells were transfected transiently with HA–MyrAkt expression vector or with vector alone. Forty-eight hours after transfection, MyrAkt was immunoprecipitated with an anti-HA antibody (HA.11). Immunoprecipitates from mock transfected (lanes 7,8) or from HA–MyrAkt transfected cells (lanes 1–6) were used for kinase reactions, as described in Materials and Methods. GST–4E-BP1 (2 μg) and histone H2B (2 μg) were used as substrates and incubated with the immunoprecipitates for the indicated times. Samples were analyzed by SDS-PAGE. An equal amount of immunoprecipitate was used for each reaction. Equal protein loading was visualized by Coomassie Blue staining. The results shown are representative of two independent experiments.
Figure 7
Akt requires FRAP activity for phosphorylation of 4E-BP1. HEK 293 MyrAkt cells were cotransfected with HA–4E-BP1 expression vector and with control vector (lane 1) or with wild-type epitope-tagged FLAG–FRAP expression vector (lanes 2–4), or a rapamycin-resistant mutant FLAG–FRAP S2035T expression vector (lane 5–7). After transfection, cells were serum deprived of for 36 hr and were either left untreated (lanes 1,2,5) or treated with wortmannin, [200 n
m
(Wort.); lanes _3,6_] or rapamycin [20 ng/ml (Rap.); lanes _4,7_]. Cell extracts were prepared, and HA–4E-BP1 was detected as described in Fig. 1A. Equal amounts of extract from the same experiment were used for detection of FLAG–FRAP with anti-Flag monoclonal antibodies. FLAG–FRAP is indicated by the large arrow. Small arrows indicate different phosphorylation states of 4E-BP1. The results shown in this figure are representative of three independent experiments.
Figure 8
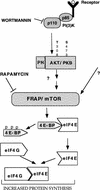
A model illustrating the signaling cascades leading to an increase in protein synthesis. PI 3-kinase, Akt, and FRAP/mTOR are downstream effectors of growth factor receptors that lead to phosphorylation of the 4E-BPs, and subsequent activation of eIF4E. For details see Discussion.
Similar articles
- Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism.
Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham C, Avruch J. Hara K, et al. J Biol Chem. 1998 Jun 5;273(23):14484-94. doi: 10.1074/jbc.273.23.14484. J Biol Chem. 1998. PMID: 9603962 - Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism.
Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, Aebersold R, Sonenberg N. Gingras AC, et al. Genes Dev. 1999 Jun 1;13(11):1422-37. doi: 10.1101/gad.13.11.1422. Genes Dev. 1999. PMID: 10364159 Free PMC article. - Signal pathways involved in activation of p70S6K and phosphorylation of 4E-BP1 following exposure of multiple myeloma tumor cells to interleukin-6.
Shi Y, Hsu JH, Hu L, Gera J, Lichtenstein A. Shi Y, et al. J Biol Chem. 2002 May 3;277(18):15712-20. doi: 10.1074/jbc.M200043200. Epub 2002 Feb 28. J Biol Chem. 2002. PMID: 11872747 - mu-Opioid receptor activates signaling pathways implicated in cell survival and translational control.
Polakiewicz RD, Schieferl SM, Gingras AC, Sonenberg N, Comb MJ. Polakiewicz RD, et al. J Biol Chem. 1998 Sep 4;273(36):23534-41. doi: 10.1074/jbc.273.36.23534. J Biol Chem. 1998. PMID: 9722592 - Insulin regulation of protein translation repressor 4E-BP1, an eIF4E-binding protein, in renal epithelial cells.
Bhandari BK, Feliers D, Duraisamy S, Stewart JL, Gingras AC, Abboud HE, Choudhury GG, Sonenberg N, Kasinath BS. Bhandari BK, et al. Kidney Int. 2001 Mar;59(3):866-75. doi: 10.1046/j.1523-1755.2001.059003866.x. Kidney Int. 2001. PMID: 11231341
Cited by
- PPM1G dephosphorylates eIF4E in control of mRNA translation and cell proliferation.
Wang P, Li Z, Kim SH, Xu H, Huang H, Yang C, Snape A, Choi JH, Bermudez S, Boivin MN, Ferry N, Karamchandani J, Nagar B, Sonenberg N. Wang P, et al. Life Sci Alliance. 2024 Aug 7;7(10):e202402755. doi: 10.26508/lsa.202402755. Print 2024 Oct. Life Sci Alliance. 2024. PMID: 39111820 Free PMC article. - Potential roles of UCH family deubiquitinases in tumorigenesis and chemical inhibitors developed against them.
Xu Z, Zhang N, Shi L. Xu Z, et al. Am J Cancer Res. 2024 Jun 15;14(6):2666-2694. doi: 10.62347/OEGE2648. eCollection 2024. Am J Cancer Res. 2024. PMID: 39005671 Free PMC article. Review. - Interplay between WNT/PI3K-mTOR axis and the microbiota in APC-driven colorectal carcinogenesis: data from a pilot study and possible implications for CRC prevention.
Di Paola FJ, Alquati C, Conti G, Calafato G, Turroni S, D'Amico F, Ceccarelli C, Buttitta F, Bernardi A, Cuicchi D, Poggioli G, Turchetti D, Ferrari S, Cannizzaro R, Realdon S, Brigidi P, Ricciardiello L. Di Paola FJ, et al. J Transl Med. 2024 Jul 5;22(1):631. doi: 10.1186/s12967-024-05305-5. J Transl Med. 2024. PMID: 38970018 Free PMC article. - eIF4A controls translation of estrogen receptor alpha and is a therapeutic target in advanced breast cancer.
Boyer JA, Sharma M, Dorso MA, Mai N, Amor C, Reiter JM, Kannan R, Gadal S, Xu J, Miele M, Li Z, Chen X, Chang Q, Pareja F, Worland S, Warner D, Sperry S, Chiang GG, Thompson PA, Yang G, Ouerfelli O, de Stanchina E, Wendel HG, Rosen EY, Chandarlapaty S, Rosen N. Boyer JA, et al. bioRxiv [Preprint]. 2024 May 11:2024.05.08.593195. doi: 10.1101/2024.05.08.593195. bioRxiv. 2024. PMID: 38766126 Free PMC article. Preprint. - Multimodal stimulation screens reveal unique and shared genes limiting T cell fitness.
Lin CP, Levy PL, Alflen A, Apriamashvili G, Ligtenberg MA, Vredevoogd DW, Bleijerveld OB, Alkan F, Malka Y, Hoekman L, Markovits E, George A, Traets JJH, Krijgsman O, van Vliet A, Poźniak J, Pulido-Vicuña CA, de Bruijn B, van Hal-van Veen SE, Boshuizen J, van der Helm PW, Díaz-Gómez J, Warda H, Behrens LM, Mardesic P, Dehni B, Visser NL, Marine JC, Markel G, Faller WJ, Altelaar M, Agami R, Besser MJ, Peeper DS. Lin CP, et al. Cancer Cell. 2024 Apr 8;42(4):623-645.e10. doi: 10.1016/j.ccell.2024.02.016. Epub 2024 Mar 14. Cancer Cell. 2024. PMID: 38490212 Free PMC article.
References
- Alessi D, James S, Downes CP, Holmes A, Gaffney P, Reese C, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7:261–269. - PubMed
- Bellacosa A, Feo DD, Godwin AK, Bell DW, Cheng JQ, Altomare DA, Wan M, Dubeau L, Scambia G, Masciullo V, Ferrandina G, Panici PB, Mancuso S, Neri G, Testa JR. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Intern J Cancer. 1995;64:280–285. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous