Hepatitis C virus core from two different genotypes has an oncogenic potential but is not sufficient for transforming primary rat embryo fibroblasts in cooperation with the H-ras oncogene - PubMed (original) (raw)
Hepatitis C virus core from two different genotypes has an oncogenic potential but is not sufficient for transforming primary rat embryo fibroblasts in cooperation with the H-ras oncogene
J Chang et al. J Virol. 1998 Apr.
Abstract
Persistent infection with hepatitis C virus (HCV) is associated with the development of liver cirrhosis and hepatocellular carcinoma. To examine the oncogenic potential of the HCV core gene product, primary rat embryo fibroblasts (REFs) were transfected with the core gene in the presence or absence of the H-ras oncogene. In contrast to a previous report (R. B. Ray, L. M. Lagging, K. Meyer, and R. Ray, J. Virol. 70:4438-4443, 1996), HCV core proteins from two different genotypes (type 1a and type 1b) were not found to transform REFs to tumorigenic phenotype in cooperation with the H-ras oncogene, although the core protein was successfully expressed 20 days after transfection. In addition, REFs transfected with E1A- but not core-expressing plasmid showed the phenotype of immortalized cells when selected with G418. The biological activity was confirmed by observing the transcription activation from two viral promoters, Rous sarcoma virus long terminal repeat and simian virus 40 promoter, which are known to be activated by the core protein from HCV-1 isolate. In contrast to the result with primary cells, the Rat-1 cell line, stably expressing HCV core protein, exhibited focus formation, anchorage-independent growth, and tumor formation in nude mice. HCV core protein was able to induce the transformation of Rat-1 cells with various efficiencies depending on the expression level of the core protein. These results indicate that HCV core protein has an oncogenic potential to transform the Rat-1 cell line but is not sufficient to either immortalize primary REFs by itself or transform primary cells in conjunction with the H-ras oncogene.
Figures
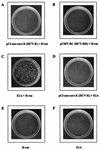
FIG. 1
The lack of transforming activity by HCV core and H-ras. E1A-plus-H-ras transfectants were photographed 14 days after transfection. The other plates were examined 30 days after transfection.
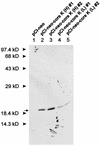
FIG. 2
Immunoblot analysis of the HCV core protein from transfected REFs. The REF transfectants were harvested 2 (lanes 1 to 4), 14 (lanes 5 and 7), or 20 (lanes 6 and 8) days after transfection, and cell lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12% polyacrylamide). The core protein was detected by immunoblotting with anti-HCV human immunoglobulin and horseradish peroxidase-conjugated anti-human immunoglobulin G. Two forms of the HCV core protein (19 and 21 kDa) are indicated by arrows. The positions of molecular mass standards are indicated.
FIG. 3
Biological activity of HCV core protein expressed in REFs. Each HCV core plasmid (2 μg) was cotransfected with a reporter plasmid (1 μg) into secondary cultures of REFs. The resulting luciferase activity, normalized to secreted alkaline phosphatase activity, is presented as relative light units.
FIG. 4
(A) Focus morphology of pCI-neo- or pCI-neo-core K-transfected Rat-1 clones. (B) Colony formation in a soft agar assay by pCI-neo- or pCI-neo-core K-transfected Rat-1 clones. H-_ras_-transformed clones are also shown as a positive control. Magnification, ×100.
FIG. 5
Immunoblot analysis of the HCV core protein from G418-selected Rat-1 clones. The experiment was performed as described in the legend to Fig. 2. pCI-neo-core K (H) (expressing the HCV-K core protein at a high level) clones express the HCV-K core protein at higher level than do pCI-neo-core K (L) clones (expressing the HCV-K core protein at a low level).
FIG. 6
Comparison of the core protein-coding sequences of the HCV-K, HCV-RH, and HCV-1 isolates. The amino acid sequence of HCV-K is shown at the top. Identical sequences are represented by dashes.
Similar articles
- Hepatitis C virus core protein cooperates with ras and transforms primary rat embryo fibroblasts to tumorigenic phenotype.
Ray RB, Lagging LM, Meyer K, Ray R. Ray RB, et al. J Virol. 1996 Jul;70(7):4438-43. doi: 10.1128/JVI.70.7.4438-4443.1996. J Virol. 1996. PMID: 8676467 Free PMC article. - The p53 tumor suppressor gene and gene product.
Levine AJ. Levine AJ. Princess Takamatsu Symp. 1989;20:221-30. Princess Takamatsu Symp. 1989. PMID: 2488233 Review. - Mechanisms of oncogene cooperation: activation and inactivation of a growth antagonist.
Ragozzino MM, Kuo A, DeGregori J, Kohl N, Ruley HE. Ragozzino MM, et al. Environ Health Perspect. 1991 Jun;93:97-103. doi: 10.1289/ehp.919397. Environ Health Perspect. 1991. PMID: 1837777 Free PMC article. Review.
Cited by
- Hepatitis B Virus X Protein Stimulates Hepatitis C Virus (HCV) Replication by Protecting HCV Core Protein from E6AP-Mediated Proteasomal Degradation.
Yoon H, Han J, Jang KL. Yoon H, et al. Microbiol Spectr. 2022 Dec 21;10(6):e0143222. doi: 10.1128/spectrum.01432-22. Epub 2022 Nov 14. Microbiol Spectr. 2022. PMID: 36374094 Free PMC article. - Chronic hepatitis C infection is associated with higher incidence of extrahepatic cancers in a Canadian population based cohort.
Darvishian M, Tang T, Wong S, Binka M, Yu A, Alvarez M, Alexander Velásquez García H, Adu PA, Jeong D, Bartlett S, Karamouzian M, Damascene Makuza J, Wong J, Ramji A, Woods R, Krajden M, Janjua N, Bhatti P. Darvishian M, et al. Front Oncol. 2022 Oct 13;12:983238. doi: 10.3389/fonc.2022.983238. eCollection 2022. Front Oncol. 2022. PMID: 36313680 Free PMC article. - Hepatitis C virus core protein activates proteasomal activator 28 gamma to downregulate p16 levels via ubiquitin-independent proteasomal degradation.
Cha S, Park I, Jang KL. Cha S, et al. Heliyon. 2021 Jan 30;7(1):e06134. doi: 10.1016/j.heliyon.2021.e06134. eCollection 2021 Jan. Heliyon. 2021. PMID: 33553768 Free PMC article. - Hepatitis C Virus: Evading the Intracellular Innate Immunity.
Ferreira AR, Ramos B, Nunes A, Ribeiro D. Ferreira AR, et al. J Clin Med. 2020 Mar 13;9(3):790. doi: 10.3390/jcm9030790. J Clin Med. 2020. PMID: 32183176 Free PMC article. Review. - Epidemiology of Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV) Related Hepatocellular Carcinoma.
Petruzziello A. Petruzziello A. Open Virol J. 2018 Feb 28;12:26-32. doi: 10.2174/1874357901812010026. eCollection 2018. Open Virol J. 2018. PMID: 29541276 Free PMC article. Review.
References
- Aach R D, Stevens C E, Hollinger F B, Mosley J W, Peterson D A, Taylor P E, Johnson R G, Barbosa L H, Nemo G J. Hepatitis C virus infection in post-transfusion hepatitis. An analysis with first- and second-generation assays. N Engl J Med. 1991;325:1325–1329. - PubMed
- Alter H J, Purcell R, Shih J, Melpolder J, Choo Q L, Kuo G. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med. 1989;321:1494–1500. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous