Cell adhesion and the integrin-linked kinase regulate the LEF-1 and beta-catenin signaling pathways - PubMed (original) (raw)
Cell adhesion and the integrin-linked kinase regulate the LEF-1 and beta-catenin signaling pathways
A Novak et al. Proc Natl Acad Sci U S A. 1998.
Abstract
The integrin-linked kinase (ILK) is an ankyrin repeat containing serine-threonine protein kinase that can interact directly with the cytoplasmic domains of the beta1 and beta3 integrin subunits and whose kinase activity is modulated by cell-extracellular matrix interactions. Overexpression of constitutively active ILK results in loss of cell-cell adhesion, anchorage-independent growth, and tumorigenicity in nude mice. We now show that modest overexpression of ILK in intestinal epithelial cells as well as in mammary epithelial cells results in an invasive phenotype concomitant with a down-regulation of E-cadherin expression, translocation of beta-catenin to the nucleus, formation of a complex between beta-catenin and the high mobility group transcription factor, LEF-1, and transcriptional activation by this LEF-1/beta-catenin complex. We also find that LEF-1 protein expression is rapidly modulated by cell detachment from the extracellular matrix, and that LEF-1 protein levels are constitutively up-regulated at ILK overexpression. These effects are specific for ILK, because transformation by activated H-ras or v-src oncogenes do not result in the activation of LEF-1/beta-catenin. The results demonstrate that the oncogenic properties of ILK involve activation of the LEF-1/beta-catenin signaling pathway, and also suggest ILK-mediated cross-talk between cell-matrix interactions and cell-cell adhesion as well as components of the Wnt signaling pathway.
Figures
Figure 1
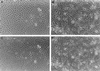
Invasion of collagen gels. Seven days after seeding cells onto collagen, ILK-14A2c6 control transfectants (A and C) and ILK-13A4a, ILK-overexpressing cells (B and D) were photographed at the surface (A and B) and beneath the surface (C and D) to visualize cells that have penetrated the gel. (×150.)
Figure 2
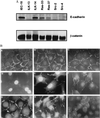
(A) Immunoblot for E-cadherin and β-catenin. Cell lysates (10 μg) were analyzed for levels of E-cadherin and β-catenin expression by Western blotting as described in Materials and Methods. (B) Indirect immunofluorescence for β-catenin. Cells were plated out on coverslips and stained with antibody toward β-catenin and then with a fluorescent secondary antibody. A) Parental IEC-18; B) control transfected ILK-14 clone A2c3; C) control transfected ILK-14 clone A2c6; D) ILK-overexpressing ILK-13 clone A4a; E) ILK-overexpressing ILK-13 clone Ala3; F) IEC-18GH3IRH kinase deficient ILK; G) IEC-18 cells expressing activated H-ras oncogene (Ras 33) and (Ras 37), respectively; H) Ras 37; I) IEC-18 cells expressing v-src oncogene (Src 2). (Bar = 5 μm.) (×1,000.)
Figure 3
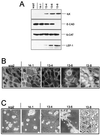
(A) ILK, E-cadherin, β-catenin, and LEF-1 expression in scp2. Nonidet P-40 cell lysates (10 μg for ILK and LEF-1; 20 μg for E-cadherin and β-catenin) were analyzed by Western blotting as described in Materials and Methods. Clone 14–1: control transfectants, transfected with antisense ILK cDNA; Clones 13–4, 13–6, and 13–8: transfected with ILK-sense cDNA. (B) Indirect immunofluorescence for β-catenin. Cells were fixed in methanol and stained with a mouse mAb for β-catenin (Transduction Laboratories) that was visualized with a fluorescent secondary antibody. (×600.) (C) Morphology on a reconstituted basement membrane gel. Cells were plated on Matrigel, maintained for 72 hr in serum-free medium, and then visualized by phase contrast microscopy. (×150.)
Figure 4
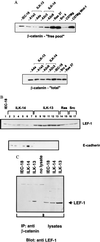
(A) Immunoblot of uncomplexed and total β-catenin. Cell lysates were precipitated with Sepharose coupled to a glutathione _S_-transferase fusion protein containing the cytoplasmic domain of E-cadherin to bind uncomplexed pools of β-catenin (19). The pelleted beads were solubilized and electrophoresed through SDS/PAGE then Western-blotted with antibody toward β-catenin. (Lower) Comparison of the total concentrations of β-catenin. C57Mg is a mouse mammary epithelial cell line and C57Mg Wnt-1 is its counterpart stably transfected to constitutively express Wnt-1 (C57805) (32). (B) Immunoblot for LEF-1 and E-cadherin. Supernatants of cells lysed in Nonidet P-40 lysis buffer (40 μg) were electrophoresed through 8% SDS/PAGE and Western-blotted with antibody toward LEF-1 and E-cadherin. Lane 1, IEC-18; lanes 2–7, ILK-14 clones A2a3, A2c3, A2c6, A2 g3, A2 g8, and A3a1; lanes 8–13, ILK-13 clones, A1a3, A1d11, A4a, A4a3, A4c, and A4i; lanes 14 and 15, Ras clones 33, 37; and lanes 16 and 17) Src clones 2,4. (C) Coimmunoprecipitation of LEF-1 with β-catenin. Cell extracts (500 μg in Nonidet P-40 lysis buffer) were immunoprecipitated with 4 μg of β-catenin mAb and electrophoresed though 8% SDS/PAGE along with 20 μg of cell lysates alone. The gel was Western-blotted with antibody toward LEF-1.
Figure 5
(A) Transcriptional activation of a LEF-1 responsive reporter construct. IEC-18 cell transfectants (described in Materials and Methods) were transiently cotransfected with a chloramphenicol acetyl transferase (CAT) reporter construct containing either wt or mutated (mut) LEF-1/TCF binding sites, and a control luciferase reporter construct. CAT activities were standardized relative to their corresponding luciferase activities. (B) Gel mobility shift assays for LEF-1. Nuclear extracts of ILK14 control transfectants (control), the kinase-deficient transfected cell line (kd), ILK-overexpressing ILK13 cell lines (wt), and Jurkat T cells, were assayed for LEF-1 (Upper, lanes 1–7) and Oct-1 (Lower, lanes 1–7) DNA binding activity, the latter to serve as a control for nuclear extract preparations. Nonspecific EBF competitor DNA (lanes 8 and 9), specific LEF-1 competitor DNA (lanes 10 and 11), anti-LEF-1 antibody (lanes 12 and 13), and anti-β-catenin antibody (lanes 16 and 17) were added to determine the specificity of the LEF-1 bandshift. A complex migrating more slowly than LEF-1 is present in both the wt ILK13 and the kinase-deficient transfected cells, and this may represent binding by another member of the LEF-1/TCF family. (C) Immunoblot showing the effect of cell detachment on the levels of LEF-1 and E-cadherin. Cells were detached from tissue culture plates with 5 mm EDTA/PBS, placed on ice (t = 0) or incubated in suspension in complete medium containing fetal calf serum at 37°C with 5% CO2 for up to 16 hr, then pelleted and extracted with Nonidet P-40 lysis buffer. Lysates (50 μg) were electrophoresed through 8% SDS/PAGE and Western-blotted with antibody toward LEF-1 and E-cadherin.
Similar articles
- Inhibition of integrin linked kinase (ILK) suppresses beta-catenin-Lef/Tcf-dependent transcription and expression of the E-cadherin repressor, snail, in APC-/- human colon carcinoma cells.
Tan C, Costello P, Sanghera J, Dominguez D, Baulida J, de Herreros AG, Dedhar S. Tan C, et al. Oncogene. 2001 Jan 4;20(1):133-40. doi: 10.1038/sj.onc.1204052. Oncogene. 2001. PMID: 11244511 - Cyr61 is overexpressed in gliomas and involved in integrin-linked kinase-mediated Akt and beta-catenin-TCF/Lef signaling pathways.
Xie D, Yin D, Tong X, O'Kelly J, Mori A, Miller C, Black K, Gui D, Said JW, Koeffler HP. Xie D, et al. Cancer Res. 2004 Mar 15;64(6):1987-96. doi: 10.1158/0008-5472.can-03-0666. Cancer Res. 2004. PMID: 15026334 - Signaling through beta-catenin and Lef/Tcf.
Novak A, Dedhar S. Novak A, et al. Cell Mol Life Sci. 1999 Oct 30;56(5-6):523-37. doi: 10.1007/s000180050449. Cell Mol Life Sci. 1999. PMID: 11212302 Free PMC article. Review. - Regulation of E-cadherin expression and beta-catenin/Tcf transcriptional activity by the integrin-linked kinase.
Oloumi A, McPhee T, Dedhar S. Oloumi A, et al. Biochim Biophys Acta. 2004 Apr 1;1691(1):1-15. doi: 10.1016/j.bbamcr.2003.12.002. Biochim Biophys Acta. 2004. PMID: 15053919 Review. - The integrin-linked kinase regulates the cyclin D1 gene through glycogen synthase kinase 3beta and cAMP-responsive element-binding protein-dependent pathways.
D'Amico M, Hulit J, Amanatullah DF, Zafonte BT, Albanese C, Bouzahzah B, Fu M, Augenlicht LH, Donehower LA, Takemaru K, Moon RT, Davis R, Lisanti MP, Shtutman M, Zhurinsky J, Ben-Ze'ev A, Troussard AA, Dedhar S, Pestell RG. D'Amico M, et al. J Biol Chem. 2000 Oct 20;275(42):32649-57. doi: 10.1074/jbc.M000643200. J Biol Chem. 2000. PMID: 10915780
Cited by
- Integrin-linked kinase (ILK) expression correlates with tumor severity in clear cell renal carcinoma.
Engelman Mde F, Grande RM, Naves MA, de Franco MF, de Paulo Castro Teixeira V. Engelman Mde F, et al. Pathol Oncol Res. 2013 Jan;19(1):27-33. doi: 10.1007/s12253-012-9554-4. Epub 2012 Jul 20. Pathol Oncol Res. 2013. PMID: 22814720 - Expression/activation of α5β1 integrin is linked to the β-catenin signaling pathway to drive migration in glioma cells.
Renner G, Noulet F, Mercier MC, Choulier L, Etienne-Selloum N, Gies JP, Lehmann M, Lelong-Rebel I, Martin S, Dontenwill M. Renner G, et al. Oncotarget. 2016 Sep 20;7(38):62194-62207. doi: 10.18632/oncotarget.11552. Oncotarget. 2016. PMID: 27613837 Free PMC article. - The roles of integrin-linked kinase in the regulation of myogenic differentiation.
Huang Y, Li J, Zhang Y, Wu C. Huang Y, et al. J Cell Biol. 2000 Aug 21;150(4):861-72. doi: 10.1083/jcb.150.4.861. J Cell Biol. 2000. PMID: 10953009 Free PMC article. - The cadherin-catenin superfamily in endocrine tumors.
Semba S, Yamakawa M, Sasano H. Semba S, et al. Endocr Pathol. 2001 Spring;12(1):1-13. doi: 10.1385/ep:12:1:01. Endocr Pathol. 2001. PMID: 11478263 Review. - MicroRNA therapeutics: design of single-stranded miR-216b mimics to target KRAS in pancreatic cancer cells.
Ferino A, Miglietta G, Picco R, Vogel S, Wengel J, Xodo LE. Ferino A, et al. RNA Biol. 2018;15(10):1273-1285. doi: 10.1080/15476286.2018.1526536. Epub 2018 Oct 11. RNA Biol. 2018. PMID: 30306823 Free PMC article.
References
- Hannigan G E, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino M G, Radeva G, Filmus J, Bell J C, Dedhar S. Nature (London) 1996;379:91–96. - PubMed
- Radeva G, Petrocelli T, Behrend E, Leung-Hagesteijn C, Filmus J, Slingerland J, Dedhar S. J Biol Chem. 1997;272:13937–13944. - PubMed
- Wu C, Keightley S Y, Leung-Hagesteijn C, Radeva G, Coppolino M, Goicoechea S, McDonald I A, Dedhar S. J Biol Chem. 1998;273:528–536. - PubMed
- Brache M E, Van Roy F M, Mareel M M. Curr Top Microbiol Immunol. 1996;213:123–161. - PubMed
- Huber O, Bierkamp C, Kemler R. Curr Opin Cell Biol. 1996;8:685–691. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous