NF-kappaB mediates alphavbeta3 integrin-induced endothelial cell survival - PubMed (original) (raw)
NF-kappaB mediates alphavbeta3 integrin-induced endothelial cell survival
M Scatena et al. J Cell Biol. 1998.
Abstract
The alphavbeta3 integrin plays a fundamental role during the angiogenesis process by inhibiting endothelial cell apoptosis. However, the mechanism of inhibition is unknown. In this report, we show that integrin-mediated cell survival involves regulation of nuclear factor-kappa B (NF-kappaB) activity. Different extracellular matrix molecules were able to protect rat aorta- derived endothelial cells from apoptosis induced by serum withdrawal. Osteopontin and beta3 integrin ligation rapidly increased NF-kappaB activity as measured by gel shift and reporter activity. The p65 and p50 subunits were present in the shifted complex. In contrast, collagen type I (a beta1-integrin ligand) did not induce NF-kappaB activity. The alphavbeta3 integrin was most important for osteopontin-mediated NF-kappaB induction and survival, since adding a neutralizing anti-beta3 integrin antibody blocked NF-kappaB activity and induced endothelial cell death when cells were plated on osteopontin. NF-kappaB was required for osteopontin- and vitronectin-induced survival since inhibition of NF-kappaB activity with nonphosphorylatable IkappaB completely blocked the protective effect of osteopontin and vitronectin. In contrast, NF-kappaB was not required for fibronectin, laminin, and collagen type I-induced survival. Activation of NF-kappaB by osteopontin depended on the small GTP-binding protein Ras and the tyrosine kinase Src, since NF-kappaB reporter activity was inhibited by Ras and Src dominant-negative mutants. In contrast, inhibition of MEK and PI3-kinase did not affect osteopontin-induced NF-kappaB activation. These studies identify NF-kappaB as an important signaling molecule in alphavbeta3 integrin-mediated endothelial cell survival.
Figures
Figure 1
Osteopontin protects endothelial cells from serum deprivation–induced cell death. Endothelial cells were plated in serum on uncoated plastic, or on plastic coated with recombinant wild-type osteopontin (OPN), or osteopontin containing a RGD to RGE mutation (OPN-RGE). Soon after spreading (∼2 h after plating), the serum was withdrawn. 48 h after plating, cells were stained with the nuclear dye Hoechst 33342, and nuclear fragmentation was assessed. In control cultures, serum was not withdrawn from the cells. Data represent the average of triplicates ± SD of a representative experiment.
Figure 2
Endothelial cells plated on osteopontin do not show nuclear fragmentation. (A and B) Cells were plated on polylysine- (A) or osteopontin- (B) coated surfaces in serum-free medium. 48 h after plating, cells were stained with the nuclear dye Hoechst 33342, and nuclear morphology was assessed by fluorescence microscope. Arrows indicate the apoptotic nuclei. Bar, 40 μM. (C) Cells were plated on polylysine- (PDL) or osteopontin- (OPN) coated surfaces in serum-free medium. Proteins were extracted 6 and 24 h after plating, electrophoresed, Western blotted, and probed with an anti-PARP antibody. Arrows indicate uncleaved (115 kD) and cleaved (85 kD) forms. (D) The relative intensity of 115 kD and 85 kD was determined with ImageQuant software (Molecular Dynamics, Inc., Sunnyvale, CA), and the percentage of cleaved product (85 kD band) was plotted.
Figure 3
αvβ3 integrin mediates osteopontin-induced endothelial cell survival. (A) Cells were plated on osteopontin-coated surfaces in serum-free medium. Soon after spreading (∼2 h after plating), F11 monoclonal antibody (triangles) or mouse IgG (squares) at the concentration of 50 μg/ml were added. Nuclear morphology was assessed at 24, 48, and 72 h. (B) Cells were plated on polylysine, osteopontin (OPN), F11, or mouse IgG (mIgG)-coated surfaces in serum-free medium. 48 h after plating, cells were stained with the nuclear dye Hoechst 33342, and nuclear morphology was assessed. Data represent the average of triplicates ± SD of a representative experiment.
Figure 4
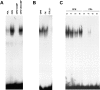
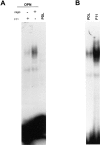
Endothelial cells plated on osteopontin have elevated NF-κB binding activity. (A) Cells were plated on polylysine (PDL) or osteopontin (OPN)-coated surfaces in serum-free medium. 4 h after plating, nuclear protein extracts were harvested, and EMSA was performed using a double-stranded 32P-labeled consensus NF-κB oligomer. Adding a 100-fold excess of unlabeled consensus NF-κB oligomer (COMP) completely inhibited binding, and a 100-fold excess of unlabeled unrelated sequence (UNCOMP) had no effect. (B) Cells were plated on osteopontin (OPN), fibronectin (FN), and collagen type I (COLL I). 4 h after plating, nuclear protein extracts were harvested, and EMSA was performed using a double-stranded 32P-labeled consensus NF-κB oligomer. (C) Cells were plated on polylysine (PDL) or osteopontin (OPN)-coated surfaces in serum-free medium. Nuclear protein extracts were harvested at 2, 4, 6, and 8 h after plating, and EMSA was performed using a double-stranded 32P-labeled consensus NF-κB oligomer.
Figure 5
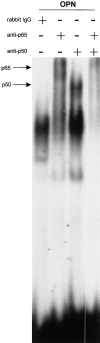
The p65 and p50 subunits form the NF-κB complex induced by osteopontin. Nuclear extract from endothelial cells plated on osteopontin were incubated with a double-stranded 32P-labeled consensus NF-κB oligomer, followed by incubation with polyclonal antibody against the p65 and p50 NF-κB subunits singly and combined, and with control rabbit IgG. Arrows indicate the shifted bands.
Figure 6
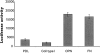
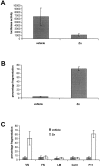
Osteopontin induces NF-κB–dependent luciferase gene expression. Endothelial cells were transfected with a NF-κB–responsive luciferase reporter construct. Cells were plated on polylysine (PDL), collagen type I (Coll type I), osteopontin (OPN), and fibronectin (FN)-coated surfaces in serum-free medium. Cell lysates were harvested, and luciferase activity was measured 8 h after plating on immobilized substrates. Data represent the average of triplicates ± SD of a representative experiment.
Figure 7
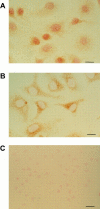
Osteopontin induces nuclear translocation of the p65 subunit. Cells were plated on osteopontin (A)- or polylysine (B)- coated surfaces in serum-free medium. 2 h after plating, cells were fixed and stained with a polyclonal anti-p65 subunit antibody. Bar, 10 μM. (C) Staining of cells plated on osteopontin with control nonimmune serum. Bar, 20 μM.
Figure 8
Osteopontin-induced NF-κB binding activity is inhibited by the β3 integrin antagonist F11. (A) Cells were plated on osteopontin-coated (OPN) surfaces in serum-free medium. Soon after spreading (∼2 h after plating), F11 monoclonal antibody or mouse-IgG (mIgG) were added at the concentration of 50 μg/ml. 10 h after plating, nuclear protein extracts were harvested, and EMSA was performed using a double-stranded 32P-labeled consensus NF-κB oligomer. (B) Cells were plated on polylysine (PDL) or immobilized F11. 4 h after plating, nuclear protein extracts were harvested, and EMSA was performed using a double-stranded 32P-labeled consensus NF-κB oligomer.
Figure 9
Inhibition of NF-κB nuclear translocation abolishes osteopontin-induced endothelial cell survival. (A) RAECΔN2 clone was transfected with a NF-κB–responsive luciferase reporter construct. Cells were then treated for 16 h with either vehicle or 100 μM of ZnSO4 (Zn) followed by a 1-h treatment with IL1-β. Cell lysates were harvested, and luciferase activity was measured. (B) RAECΔN2 clone was plated on osteopontin-coated surfaces in serum-free medium in the absence or presence of 20 μM ZnSO4 (Zn). 16 h later, cells were stained with the nuclear dye Hoechst 33342, and nuclear fragmentation was assessed. (C) RAECΔN2 clone was plated on vitronectin (VN), fibronectin (FN), laminin (LM), collagen type I (Coll I), and F11 monoclonal antibody–coated surfaces in serum-free medium in the absence (vehicle) or presence of 20 μM ZnSO4 (Zn). 16 h later, cells were stained with the nuclear dye Hoechst 33342, and nuclear fragmentation was assessed. Data represent the average of triplicates ± SD of a representative experiment. Every experiment was repeated with a separate clone (RAECΔN5), and identical results were obtained.
Figure 10
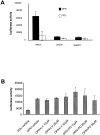
Ras and Src mediate osteopontin-induced NF-kB activation. (A) Cells were cotransfected with the NF-κB–responsive luciferase reporter construct and either the RasN17 construct, the Src kinase dead (SrcDN) construct, or vector alone. Cells were then plated on polylysine (PDL)- or osteopontin (OPN)-coated surfaces in serum-free medium. Cell lysates were harvested, and luciferase activity was measured 8 h after plating on immobilized substrates. (B) Cells were transfected with the NF-κB–responsive luciferase reporter construct. Cells were then plated on polylysine (PDL)- or osteopontin (OPN)-coated surfaces in serum-free medium. Cells plated on osteopontin were treated with the indicated concentrations of LY-294002 and PD98059 compounds. Cell lysates were harvested, and luciferase activity was measured 8 h after plating. Data represent the average of triplicates ± SD of a representative experiment.
Similar articles
- Molecular mediators of alphavbeta3-induced endothelial cell survival.
Rice J, Courter DL, Giachelli CM, Scatena M. Rice J, et al. J Vasc Res. 2006;43(5):422-36. doi: 10.1159/000094884. Epub 2006 Aug 3. J Vasc Res. 2006. PMID: 16888388 - Src kinase activity is required for integrin alphaVbeta3-mediated activation of nuclear factor-kappaB.
Courter DL, Lomas L, Scatena M, Giachelli CM. Courter DL, et al. J Biol Chem. 2005 Apr 1;280(13):12145-51. doi: 10.1074/jbc.M412555200. Epub 2005 Jan 28. J Biol Chem. 2005. PMID: 15695822 - Osteoprotegerin is an alpha vbeta 3-induced, NF-kappa B-dependent survival factor for endothelial cells.
Malyankar UM, Scatena M, Suchland KL, Yun TJ, Clark EA, Giachelli CM. Malyankar UM, et al. J Biol Chem. 2000 Jul 14;275(28):20959-62. doi: 10.1074/jbc.C000290200. J Biol Chem. 2000. PMID: 10811631 - Distinctive role of integrin-mediated adhesion in TNF-induced PKB/Akt and NF-kappaB activation and endothelial cell survival.
Bieler G, Hasmim M, Monnier Y, Imaizumi N, Ameyar M, Bamat J, Ponsonnet L, Chouaib S, Grell M, Goodman SL, Lejeune F, Rüegg C. Bieler G, et al. Oncogene. 2007 Aug 23;26(39):5722-32. doi: 10.1038/sj.onc.1210354. Epub 2007 Mar 19. Oncogene. 2007. PMID: 17369858 - Kaempferol inhibits the production of ROS to modulate OPN-αvβ3 integrin pathway in HUVECs.
Xiao HB, Lu XY, Liu ZK, Luo ZF. Xiao HB, et al. J Physiol Biochem. 2016 Jun;72(2):303-13. doi: 10.1007/s13105-016-0479-3. Epub 2016 Mar 21. J Physiol Biochem. 2016. PMID: 27000882
Cited by
- Vitronectin-activated αvβ3 and αvβ5 integrin signalling specifies haematopoietic fate in human pluripotent stem cells.
Shen J, Zhu Y, Zhang S, Lyu S, Lyu C, Feng Z, Hoyle DL, Wang ZZ, Cheng T. Shen J, et al. Cell Prolif. 2021 Apr;54(4):e13012. doi: 10.1111/cpr.13012. Epub 2021 Mar 3. Cell Prolif. 2021. PMID: 33656760 Free PMC article. - Lunasin as a Promising Plant-Derived Peptide for Cancer Therapy.
Alves de Souza SM, Hernández-Ledesma B, de Souza TLF. Alves de Souza SM, et al. Int J Mol Sci. 2022 Aug 23;23(17):9548. doi: 10.3390/ijms23179548. Int J Mol Sci. 2022. PMID: 36076946 Free PMC article. Review. - Osteopontin induces growth of metastatic tumors in a preclinical model of non-small lung cancer.
Shojaei F, Scott N, Kang X, Lappin PB, Fitzgerald AA, Karlicek S, Simmons BH, Wu A, Lee JH, Bergqvist S, Kraynov E. Shojaei F, et al. J Exp Clin Cancer Res. 2012 Mar 23;31(1):26. doi: 10.1186/1756-9966-31-26. J Exp Clin Cancer Res. 2012. PMID: 22444159 Free PMC article. - Microglia-Derived Spp1 Promotes Pathological Retinal Neovascularization via Activating Endothelial Kit/Akt/mTOR Signaling.
Bai Q, Wang X, Yan H, Wen L, Zhou Z, Ye Y, Jing Y, Niu Y, Wang L, Zhang Z, Su J, Chang T, Dou G, Wang Y, Sun J. Bai Q, et al. J Pers Med. 2023 Jan 11;13(1):146. doi: 10.3390/jpm13010146. J Pers Med. 2023. PMID: 36675807 Free PMC article. - Alpha 5 beta 1 integrin activates an NF-kappa B-dependent program of gene expression important for angiogenesis and inflammation.
Klein S, de Fougerolles AR, Blaikie P, Khan L, Pepe A, Green CD, Koteliansky V, Giancotti FG. Klein S, et al. Mol Cell Biol. 2002 Aug;22(16):5912-22. doi: 10.1128/MCB.22.16.5912-5922.2002. Mol Cell Biol. 2002. PMID: 12138201 Free PMC article.
References
- Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. - PubMed
- Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. - PubMed
- Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F31 CA070131/CA/NCI NIH HHS/United States
- HL-18645/HL/NHLBI NIH HHS/United States
- DK-47659/DK/NIDDK NIH HHS/United States
- R01 HL052585/HL/NHLBI NIH HHS/United States
- HL-52585/HL/NHLBI NIH HHS/United States
- T32 CA009437/CA/NCI NIH HHS/United States
- P01 HL018645/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous