Up-regulation of inducible nitric oxide synthase expression in cancer-prone p53 knockout mice - PubMed (original) (raw)
Up-regulation of inducible nitric oxide synthase expression in cancer-prone p53 knockout mice
S Ambs et al. Proc Natl Acad Sci U S A. 1998.
Abstract
High concentrations of nitric oxide (NO) cause DNA damage and apoptosis in many cell types. Thus, regulation of NO synthase (NOS) activity is essential for minimizing effects of cytotoxic and genotoxic nitrogen oxide species. We have shown previously that NO-induced p53 protein accumulation down-regulates basal and cytokine-modulated inducible NOS (NOS2) expression in human cells in vitro. To further characterize the feedback loop between NOS2 and p53, we have investigated NO production, i.e., urinary nitrate plus nitrite excretion, and NOS2 expression in homozygous p53 knockout (KO) mice. We report here that untreated p53 KO mice excreted 70% more nitrite plus nitrate than mice with wild-type (wt) p53. NOS2 protein expression was constitutively detected in the spleen of untreated p53 KO mice, whereas it was undetectable in the spleen of wt p53 controls. Upon treatment with heat-inactivated Corynebacterium parvum, urinary nitrite plus nitrate excretion of p53 KO mice exceeded that of wt controls by approximately 200%. C. parvum treatment also induced p53 accumulation in the liver. Splenectomy reduced the NO output of C. parvum-treated p53 KO mice but not of wt p53 controls. Although NO production and NOS2 protein expression were increased similarly in KO and wt p53 mice 10 days after injection of C. parvum, NOS2 expression returned to baseline levels only in wt p53 controls while remaining up-regulated in p53 KO mice. These genetic and functional data indicate that p53 is an important transrepressor of NOS2 expression in vivo and attenuates excessive NO production in a regulatory negative feedback loop.
Figures
Figure 1
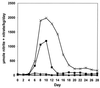
Urinary nitrite plus nitrate excretion is increased in p53 KO mice. Wt p53 (■) and p53 KO (×) mice were treated with C. parvum (100 mg/kg at day 0) or the same volume of 0.9% NaCl [wt p53 (♦), p53 KO (▴)], and daily urine samples were collected (four mice per treatment group). The total urinary nitrite plus nitrate excretion of _C. parvum_-treated p53 KO mice was approximately 200% higher than the output of wt p53 mice. Although urinary nitrite plus nitrate excretion of wt p53 mice returned to baseline levels after 14 days, p53 KO mice maintained an elevated excretion throughout the 28 days of urine collection.
Figure 2
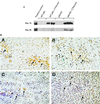
(A) Sustained NOS2 protein expression in liver of p53 KO mice after C. parvum treatment. Liver tissues from _C. parvum_-treated (100 mg/kg) wt p53 and p53 KO mice were analyzed for NOS2 expression by Western blotting (100 μg protein per lane); tissue extracts of two animals per group were analyzed. NOS2 protein levels were undetectable in controls, and C. parvum treatment produced higher levels of NOS2 protein in p53 KO mice than wt p53 mice. NOS2 protein remained markedly increased in p53 KO mice 28 days after treatment, whereas NOS2 expression returned to undetectable levels in mice with wt p53. (B) NOS2 immunostaining in the liver and spleen of wt p53 (A and C) and p53 KO (B and D) mice 10 days after i.p. injection of C. parvum (100 mg/kg). In the liver (A and B, ×400), C. parvum treatment produced cytoplasmic NOS2 staining of hepatocytes localized in focal clusters not strongly related to the lobular architecture. In addition, scattered mononuclear cells consistent with lymphocytes were stained. Cirrhosis was not evident, and there was no significant difference in damage between the wt and KO mice. Spleen (C and D, ×400): NOS2 immunostains produced intense staining of lymphocytes, which occurred predominantly in clusters distributed outside the germinal centers. Similar staining patterns occurred in both wt and KO mice. Counterstain: hematoxylin/eosin.
Figure 3
C. parvum treatment induces wt p53 accumulation in mouse liver. Liver protein extracts of wt p53 mice were prepared 10 and 28 days after injection of C. parvum (100 mg/kg); tissue extracts of two animals per group were analyzed. p53 protein was immunoprecipitated by using 300 μg of protein extract and 10 μl of a polyclonal p53 antiserum (CM-1) and was detected with the monoclonal antibody PAb 246. p53 is highly expressed 10 and 28 days after the treatment with C. parvum.
Figure 4
Increased splenic NOS2 expression of p53 KO mice. p53 KO and wt p53 mice were treated with C. parvum (100 mg/kg), and splenic extracts of untreated and _C. parvum_-treated animals were prepared 21 days later. NOS2 protein expression was measured by Western blotting (300 μg protein per lane) in two tissue extracts per treatment group. Substantial levels of NOS2 protein are expressed in p53 KO mice with or without C. parvum treatment, but it is undetectable in wt p53 mice.
Figure 5
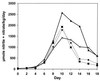
Splenectomy reduces _C. parvum_-stimulated nitrite plus nitrate excretion in p53 KO mice but not in mice with wt p53. Splenectomized and intact wt p53 (■, ♦) and p53 KO mice (×, ▴) were treated with C. parvum (100 mg/kg at day zero), and the total urinary nitrate plus nitrite excretion was measured. Splenectomy (×) reduced the urinary nitrite plus nitrate excretion of p53 KO mice by 30–40% when compared with the intact KO mice (▴). Splenectomy (■) did not affect the urinary excretion of nitrite plus nitrate in mice with wt p53 (intact = ♦). Each group contained four animals.
Similar articles
- Inflammation regulates microRNA expression in cooperation with p53 and nitric oxide.
Mathé E, Nguyen GH, Funamizu N, He P, Moake M, Croce CM, Hussain SP. Mathé E, et al. Int J Cancer. 2012 Aug 1;131(3):760-5. doi: 10.1002/ijc.26403. Epub 2011 Nov 1. Int J Cancer. 2012. PMID: 22042537 Free PMC article. - Nitric oxide-induced p53 accumulation and regulation of inducible nitric oxide synthase expression by wild-type p53.
Forrester K, Ambs S, Lupold SE, Kapust RB, Spillare EA, Weinberg WC, Felley-Bosco E, Wang XW, Geller DA, Tzeng E, Billiar TR, Harris CC. Forrester K, et al. Proc Natl Acad Sci U S A. 1996 Mar 19;93(6):2442-7. doi: 10.1073/pnas.93.6.2442. Proc Natl Acad Sci U S A. 1996. PMID: 8637893 Free PMC article. - The spinal cord expression of neuronal and inducible nitric oxide synthases and their contribution in the maintenance of neuropathic pain in mice.
Hervera A, Negrete R, Leánez S, Martín-Campos JM, Pol O. Hervera A, et al. PLoS One. 2010 Dec 13;5(12):e14321. doi: 10.1371/journal.pone.0014321. PLoS One. 2010. PMID: 21179208 Free PMC article. - Regulation of the expression of the inflammatory nitric oxide synthase (NOS2) by cyclic AMP.
Galea E, Feinstein DL. Galea E, et al. FASEB J. 1999 Dec;13(15):2125-37. doi: 10.1096/fasebj.13.15.2125. FASEB J. 1999. PMID: 10593859 Review. - Candidate pathways linking inducible nitric oxide synthase to a basal-like transcription pattern and tumor progression in human breast cancer.
Ambs S, Glynn SA. Ambs S, et al. Cell Cycle. 2011 Feb 15;10(4):619-24. doi: 10.4161/cc.10.4.14864. Epub 2011 Feb 15. Cell Cycle. 2011. PMID: 21293193 Free PMC article. Review.
Cited by
- Identifying distinctive tissue and fecal microbial signatures and the tumor-promoting effects of deoxycholic acid on breast cancer.
Wang N, Yang J, Han W, Han M, Liu X, Jiang L, Cao H, Jing M, Sun T, Xu J. Wang N, et al. Front Cell Infect Microbiol. 2022 Dec 13;12:1029905. doi: 10.3389/fcimb.2022.1029905. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36583106 Free PMC article. - Pro-Tumor Activity of Endogenous Nitric Oxide in Anti-Tumor Photodynamic Therapy: Recently Recognized Bystander Effects.
Girotti AW, Bazak J, Korytowski W. Girotti AW, et al. Int J Mol Sci. 2023 Jul 17;24(14):11559. doi: 10.3390/ijms241411559. Int J Mol Sci. 2023. PMID: 37511317 Free PMC article. Review. - Gene expression profiles of NO- and HNO-donor treated breast cancer cells: insights into tumor response and resistance pathways.
Cheng RY, Basudhar D, Ridnour LA, Heinecke JL, Kesarwala AH, Glynn S, Switzer CH, Ambs S, Miranda KM, Wink DA. Cheng RY, et al. Nitric Oxide. 2014 Dec 1;43:17-28. doi: 10.1016/j.niox.2014.08.003. Epub 2014 Aug 19. Nitric Oxide. 2014. PMID: 25153034 Free PMC article. Review. - Nitric oxide-mediated resistance to photodynamic therapy in a human breast tumor xenograft model: Improved outcome with NOS2 inhibitors.
Fahey JM, Girotti AW. Fahey JM, et al. Nitric Oxide. 2017 Jan 30;62:52-61. doi: 10.1016/j.niox.2016.12.003. Epub 2016 Dec 19. Nitric Oxide. 2017. PMID: 28007662 Free PMC article. - Hypoxic inducible factor 1alpha, extracellular signal-regulated kinase, and p53 are regulated by distinct threshold concentrations of nitric oxide.
Thomas DD, Espey MG, Ridnour LA, Hofseth LJ, Mancardi D, Harris CC, Wink DA. Thomas DD, et al. Proc Natl Acad Sci U S A. 2004 Jun 15;101(24):8894-9. doi: 10.1073/pnas.0400453101. Epub 2004 Jun 3. Proc Natl Acad Sci U S A. 2004. PMID: 15178764 Free PMC article.
References
- Crow J P, Beckman J S. Adv Pharmacol. 1995;34:17–43. - PubMed
- Wink D A, Hanbauer I, Grisham M B, Laval F, Nims R W, Laval J, Cook J, Pacelli R, Liebmann J, Krishna M, et al. Curr Top Cell Reg. 1996;34:159–187. - PubMed
- Tamir S, Tannenbaum S R. Biochim Biophys Acta. 1996;1288:F31–F36. - PubMed
- Wink D A, Kasprzak K S, Maragos C M, Elespuru R K, Misra M, Dunams T M, Cebula T A, Koch W H, Andrews A W, Allen J S, Keefer L K. Science. 1991;254:1001–1003. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous