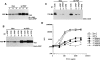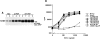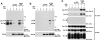Cooperative inhibition of T-cell antigen receptor signaling by a complex between a kinase and a phosphatase - PubMed (original) (raw)
Cooperative inhibition of T-cell antigen receptor signaling by a complex between a kinase and a phosphatase
J F Cloutier et al. J Exp Med. 1999.
Abstract
Antigen receptor-triggered T-cell activation is mediated by the sequential action of the Src and Syk/Zap-70 families of protein tyrosine kinases (PTKs). Previously, we reported that another PTK termed p50(csk) was a potent negative regulator of T-cell receptor (TCR) signaling because of its ability to inactivate Src-related kinases. This inhibitory effect required the catalytic activity of Csk, as well as its Src homology (SH)3 and SH2 domains. Subsequent studies uncovered that, via its SH3 domain, p50(csk) was associated with PEP, a proline-enriched protein tyrosine phosphatase (PTP) of unknown function expressed in hemopoietic cells. Herein, we have attempted to identify the role of the Csk-PEP complex in T lymphocytes. The results of our experiments showed that, like Csk, PEP was a strong repressor of TCR signaling. This property was dependent on the phosphatase activity of PEP, as well as on the sequence mediating its binding to p50(csk). Through reconstitution experiments in Cos-1 cells, evidence was obtained that Csk and PEP act synergistically to inhibit protein tyrosine phosphorylation by Src-related kinases, and that this effect requires their association. Finally, experiments with a substrate-trapping mutant of PEP suggested that PEP functions by dephosphorylating and inactivating the PTKs responsible for T-cell activation. In addition to giving novel insights into the mechanisms involved in the negative regulation of T-cell activation, these findings indicate that the association of an inhibitory PTK with a PTP constitutes a more efficient means of inhibiting signal transduction by Src family kinases in vivo.
Figures
Figure 1
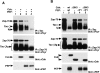
Effect of PEP on TCR-mediated lymphokine secretion. (A) Expression of PEP in BI-141 T cells. The abundance of PEP in representative cell lines was determined by anti-PEP immunoblotting of anti-PEP immunoprecipitates. Lane 1, Neo.1; lane 2, Neo.3; lane 3, Neo.5; lane 4, wt PEP.68; lane 5, wt PEP.78; and lane 6, wt PEP.82. The position of a molecular mass marker is shown on the right, whereas that of PEP is indicated on the left. Exposure: 10 h. (B) Association of PEP with Csk. The association of PEP with Csk was evaluated by immunoblotting of anti-Csk immunoprecipitates with an anti-PEP serum. Lanes 1 and 2, Neo.1; lanes 3 and 4, Neo.3; lanes 5 and 6, wt PEP.68; and lanes 7 and 8, wt PEP.78. The migration of a molecular weight marker is shown on the right; that of PEP is indicated on the left. Exposure: 18 h. (C) Association of Csk with PEP. The association of Csk with PEP was determined by immunoblotting of anti-PEP immunoprecipitates with anti-Csk antibodies. Lanes 1–4, Neo.5; and lanes 5–8, wt PEP.78. The migration of Csk is indicated on the left. Exposure: 45 s (enhanced chemiluminescence). (D) TCR-mediated lymphokine secretion. Cells were stimulated for 24 h with the indicated concentrations of anti-TCR MAb F23.1 (abscissa) coated on plastic. Lymphokine production was assayed using the IL-2–dependent indicator cell line HT-2. Thymidine incorporation is shown on the ordinate in cpm. All assays were done in triplicate.
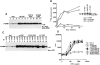
Figure 2
Role of phosphatase activity of PEP. (A) Expression of catalytically defective PEP mutants. The expression levels of PEP in representative cell lines containing C227S PEP or R233M PEP were determined as for Fig. 1 A. Lane 1, Neo.1; lane 2, Neo.3; lane 3, Neo.5; lane 4, wt PEP.68; lane 5, wt PEP.78; lane 6, wt PEP.82; lane 7, C227S PEP.19; lane 8, C227S PEP.23; lane 9, C227S PEP.28; lane 10, R233M PEP.1; and lane 11, R233M PEP.47. The position of a molecular mass marker is shown on the right, whereas that of PEP is indicated on the left. Exposure: 14 h. (B) Tyrosine phosphatase activity of PEP mutants. The catalytic activity of the PEP mutants was measured by immune complex phosphatase assay using radiolabeled myelin basic protein as a substrate. Immune complexes were incubated with myelin basic protein for the indicated periods of time (abscissa). Release of inorganic phosphate was measured by scintillation counting and is shown on the ordinate (cpm). The abundance of the various PEP mutants was also monitored by anti-PEP immunoblotting of parallel anti-PEP immunoprecipitates (inset). The position of PEP is indicated on the left. Exposure: 12 h. (C) Association of PEP mutants with Csk. The ability of the PEP mutants to coimmunoprecipitate with Csk was assayed as outlined for Fig. 1 B. Lanes 1 and 2, Neo.1; lanes 3 and 4, Neo.3; lanes 5 and 6, wt PEP.68; lanes 7 and 8, wt PEP.82; lanes 9 and 10, C227S PEP.19; lanes 11 and 12, C227S PEP.23; lanes 13 and 14, ΔP1 PEP.3; and lanes 15 and 16, ΔP1 PEP.29. The migration of a molecular weight marker is indicated on the right, while that of PEP is shown on the left. Exposure: 16 h. (D) Lymphokine production assay. Assay was carried out as described for Fig. 1 D.
Figure 3
Importance of Csk-binding domain of PEP. (A) Expression of ΔP1 PEP. The abundance of PEP in representative cell lines expressing ΔP1 PEP was evaluated as described in the legend of Fig. 1 A. Lane 1, Neo.1; lane 2, Neo.3; lane 3, wt PEP.68; lane 4, wt PEP.78; lane 5, wt PEP.82; lane 6, ΔP1 PEP.3; lane 7, ΔP1 PEP.7; lane 8, ΔP1 PEP.29; and lane 9, ΔP1 PEP.34. The position of a molecular mass marker is shown on the right, whereas that of PEP is indicated on the left. Exposure: 10 h. (B) Lymphokine production assay. Assay was carried out as described for Fig. 1 D.
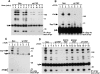
Figure 4
Impact of PEP on TCR-mediated protein tyrosine phosphorylation. (A) Overall protein tyrosine phosphorylation. Cells were stimulated for the indicated times with biotinylated anti-TCR mAb F23.1 and avidin. After cell lysis, tyrosine phosphorylated proteins were immunoprecipitated with antiphosphotyrosine (αP.tyr) antibodies and revealed by subsequent antiphosphotyrosine immunoblotting. Lanes 1–3, Neo.1; lanes 4–6, Neo.3; lanes 7–9, wt PEP.68; and lanes 10–12, wt PEP.78. The migrations of prestained molecular mass markers are indicated on the right, while that of the H chain (Ig) is shown on the left. Exposure: 16 h. (B) Tyrosine phosphorylation of Zap-70. Cells were activated as outlined for A, except that stimulation was for 2 min only. Tyrosine phosphorylation of Zap-70 was monitored by immunoblotting of anti–Zap-70 immunoprecipitates with antiphosphotyrosine antibodies. Lanes 1 and 2, Neo.1; lanes 3 and 4, Neo.3; lanes 5 and 6, wt PEP.68; and lanes 7 and 8, wt PEP.78. The migrations of prestained molecular weight markers are shown on the right; those of c-Cbl, Zap-70, and H chain (Ig) are indicated on the left. Exposure: 24 h. (C) Tyrosine phosphorylation of ζ. Experiment was as for B, except that the ζ subunit of TCR was immunoprecipitated with anti-ζ mAb H146. Lanes 1 and 2, Neo.1; lanes 3 and 4, Neo.3; lanes 5 and 6, wt PEP.68; and lanes 7 and 8, wt PEP.78. The migrations of prestained molecular mass markers are indicated on the right; those of Zap-70, H chain (Ig), and ζ (zeta) are shown on the left. Exposure: 12 h (using a PhosphorImager). (D) Effect of PEP mutants on TCR-mediated protein tyrosine phosphorylation. Experiment was as outlined for A, except that cells expressing the various PEP mutants were studied. Cells were stimulated for 2 min. Lanes 1 and 2, Neo.1; lanes 3 and 4, Neo.3; lanes 5 and 6, wt PEP.68; lanes 7 and 8, wt PEP.78; lanes 9 and 10, C227S PEP.28; lanes 11 and 12, R233M PEP.1; lanes 13 and 14, R233M PEP.47; lanes 15 and 16, ΔP1 PEP.3; and lanes 17 and 18, ΔP1 PEP.29. The positions of prestained molecular weight markers are shown on the right, whereas that of H chain (Ig) is indicated on the left. Exposure: 16 h.
Figure 5
Cooperative inhibition of protein tyrosine phosphorylation by Csk and PEP in Cos-1 cells. (A) Effects of wild-type Csk and PEP on protein tyrosine phosphorylation in Cos-1 cells. Cos-1 cells were transiently transfected with cDNAs coding for Tac-ζ, wild-type FynT, and kinase-inactive Zap-70, in the absence or presence of Csk and/or PEP. Tyrosine phosphorylation of Tac-ζ (top panel) and Zap-70 (second panel) was examined by immunoblotting of either anti-Tac mAb 7G7 or anti– Zap-70 immunoprecipitates with antiphosphotyrosine antibodies. The expression of Csk (third panel), and PEP (bottom panel) was monitored by immunoblotting of total cell lysates with the indicated antisera. Equivalent expression of Tac-ζ, FynT, and Zap-70 in the transfected cell populations was confirmed by immunoblotting of cell lysates with the appropriate antibodies (data not shown). The migrations of Zap-70, Tac-ζ, H chain (Ig), Csk, and PEP are indicated on the left. Exposures: 6 h. (B) Effects of ΔSH3 Csk and ΔP1 PEP on protein tyrosine phosphorylation in Cos-1 cells. Experiment was as in A, except that cDNAs coding for ΔSH3 Csk and ΔP1 PEP were used. Equivalent levels of Tac-ζ, FynT, and Zap-70 were demonstrated by immunoblotting of total cell lysates with the appropriate antibodies (data not shown). The migrations of Zap-70, Tac-ζ, H chain (Ig), Csk, and PEP are indicated on the left. Exposures: 5 h, except for the bottom panel (7 h).
Figure 6
Identification of potential PEP substrates by substrate trapping. (A) Effect of D195A PEP on protein tyrosine phosphorylation in Cos-1 cells. Cos-1 cells were transiently transfected as for Fig. 5 A. Appropriate levels of expression of Tac-ζ, FynT, Zap-70, and Csk were documented by immunoblotting of total cell lysates with the appropriate antibodies (data not shown). The migrations of Zap-70, Tac-ζ and PEP are indicated on the left, whereas those of prestained molecular weight markers are shown on the right. Exposures: 13 h. (B) Association of tyrosine phosphorylated proteins with D195A PEP. Lysates from the experiment shown in A were immunoprecipitated with anti-PEP antibodies and probed by immunoblotting with either antiphosphotyrosine (αP.tyr; top panel) or anti-PEP antibodies (bottom panel). The positions of the 70- and 59-kD tyrosine phosphorylated proteins are indicated on the left, whereas those of prestained molecular weight markers are shown on the right. Exposures: top panel, 13 h; bottom panel, 7 h. (C) Identification of tyrosine phosphorylated proteins associated with D195A PEP. Experiment was as in A and B, except that PEP immunoprecipitates and total cell lysates were immunoblotted with anti–Zap-70, anti-Fyn, or anti-ζ antibodies. The positions of Zap-70, FynT, and Tac-ζ are shown on the left. Exposures: top three panels, 48 h; bottom three panels, 13 h.
Figure 7
Peptide mapping studies. (A) Immunoprecipitations. Cells were metabolically labeled with 32Pi, as outlined in Materials and Methods. After lysis, FynT polypeptides were recovered by immunoprecipitation (IP) and analyzed by SDS-PAGE. The positions of prestained molecular mass markers are shown on the right. Those of the 59- and 65-kD species of FynT (FynT*) are indicated on the left. Exposure: 13 h. (B) Cyanogen bromide cleavage. Radiolabeled FynT was cleaved with cyanogen bromide and the resulting fragments were resolved in 18% SDS-PAGE gels. Phosphorylated products were detected by autoradiography. To allow better identification of the fragment (C2) containing the site of autophosphorylation of FynT, tyrosine 417, a peptide map of radiolabeled F528 FynT from cells treated with pervanadate is shown in lane 5. Under these conditions, the mutant FynT protein is extensively phosphorylated at tyrosine 417. The presence of a phosphorylated C3 fragment in this lane was due to contamination of the immunoprecipitates with endogenous Fyn molecules from Cos-1 cells. The positions of C1 (which contains NH2-terminal sites of serine and threonine phosphorylation), C2 (which contains tyrosine 417), and C3 (which bears tyrosine 528) are shown on the left. The migrations of prestained molecular mass markers are indicated on the right. Exposure: Lanes 1, 2, and 5, 3.5 h; lanes 3 and 4, 9 h.
Similar articles
- SH2 domain-mediated interaction of inhibitory protein tyrosine kinase Csk with protein tyrosine phosphatase-HSCF.
Wang B, Lemay S, Tsai S, Veillette A. Wang B, et al. Mol Cell Biol. 2001 Feb;21(4):1077-88. doi: 10.1128/MCB.21.4.1077-1088.2001. Mol Cell Biol. 2001. PMID: 11158295 Free PMC article. - Inhibitory tyrosine protein kinase p50csk is associated with protein-tyrosine phosphatase PTP-PEST in hemopoietic and non-hemopoietic cells.
Davidson D, Cloutier JF, Gregorieff A, Veillette A. Davidson D, et al. J Biol Chem. 1997 Sep 12;272(37):23455-62. doi: 10.1074/jbc.272.37.23455. J Biol Chem. 1997. PMID: 9287362 - Positive and negative regulation of T-cell activation through kinases and phosphatases.
Mustelin T, Taskén K. Mustelin T, et al. Biochem J. 2003 Apr 1;371(Pt 1):15-27. doi: 10.1042/BJ20021637. Biochem J. 2003. PMID: 12485116 Free PMC article. Review. - Negative regulation of antigen receptor signaling in lymphocytes.
Plas DR, Thomas ML. Plas DR, et al. J Mol Med (Berl). 1998 Jul;76(8):589-95. doi: 10.1007/s001090050254. J Mol Med (Berl). 1998. PMID: 9694436 Review.
Cited by
- Meta-analysis of the family-based association between the PTPN22 C1858T polymorphism and type 1 diabetes.
Lee YH, Song GG. Lee YH, et al. Mol Biol Rep. 2013 Jan;40(1):211-5. doi: 10.1007/s11033-012-2051-8. Epub 2012 Oct 8. Mol Biol Rep. 2013. PMID: 23054006 - SH2 domain-mediated interaction of inhibitory protein tyrosine kinase Csk with protein tyrosine phosphatase-HSCF.
Wang B, Lemay S, Tsai S, Veillette A. Wang B, et al. Mol Cell Biol. 2001 Feb;21(4):1077-88. doi: 10.1128/MCB.21.4.1077-1088.2001. Mol Cell Biol. 2001. PMID: 11158295 Free PMC article. - Constitutive activation of Lck and Fyn tyrosine kinases in large granular lymphocytes infected with the gamma-herpesvirus agents of malignant catarrhal fever.
Swa S, Wright H, Thomson J, Reid H, Haig D. Swa S, et al. Immunology. 2001 Jan;102(1):44-52. doi: 10.1046/j.1365-2567.2001.01154.x. Immunology. 2001. PMID: 11168636 Free PMC article. - Do non-HLA genes influence development of persistent islet autoimmunity and type 1 diabetes in children with high-risk HLA-DR,DQ genotypes?
Steck AK, Zhang W, Bugawan TL, Barriga KJ, Blair A, Erlich HA, Eisenbarth GS, Norris JM, Rewers MJ. Steck AK, et al. Diabetes. 2009 Apr;58(4):1028-33. doi: 10.2337/db08-1179. Epub 2009 Feb 2. Diabetes. 2009. PMID: 19188433 Free PMC article. - CD45, CD148, and Lyp/Pep: critical phosphatases regulating Src family kinase signaling networks in immune cells.
Hermiston ML, Zikherman J, Zhu JW. Hermiston ML, et al. Immunol Rev. 2009 Mar;228(1):288-311. doi: 10.1111/j.1600-065X.2008.00752.x. Immunol Rev. 2009. PMID: 19290935 Free PMC article. Review.
References
- Xu W, Harrison SC, Eck MJ. Three-dimensional structure of the tyrosine kinase c-Src. Nature. 1997;385:595–602. - PubMed
- Sicheri F, Moarefi I, Kuriyan J. Crystal structure of the Src family tyrosine kinase Hck. Nature. 1997;385:602–609. - PubMed
- Moarefi I, LaFevre-Bernt M, Sicheri F, Huse M, Lee C-H, Kuriyan J, Miller WT. Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement. Nature. 1997;385:650–653. - PubMed
- Nada S, Okada M, MacAuley A, Cooper JA, Nakagawa H. Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src . Nature. 1991;351:69–72. - PubMed
- Chow LML, Veillette A. The Src and Csk families of protein tyrosine kinases in hemopoietic cells. Semin Immunol. 1995;7:207–226. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous