Molybdenum (original) (raw)
Data Zone | Discovery | Facts | Appearance & Characteristics | Uses | Abundance & Isotopes | References
The chemical element molybdenum is classed as a transition metal. It was discovered in 1778 by Carl W. Scheele.

Data Zone
| Classification: | Molybdenum is a transition metal |
|---|---|
| Color: | silvery-white |
| Atomic weight: | 95.94 |
| State: | solid |
| Melting point: | 2623 oC, 2896 K |
| Boiling point: | 4640 oC, 4913 K |
| Electrons: | 42 |
| Protons: | 42 |
| Neutrons in most abundant isotope: | 56 |
| Electron shells: | 2,8,18,13,1 |
| Electron configuration: | [Kr] 4d5 5s1 |
| Density @ 20oC: | 10.2 g/cm3 |
Show more, including: Heats, Energies, Oxidation,
Reactions, Compounds, Radii, Conductivities
| Atomic volume: | 9.4 cm3/mol |
|---|---|
| Structure: | bcc: body-centered cubic |
| Hardness: | 5.5 mohs |
| Specific heat capacity | 0.25 J g-1 K-1 |
| Heat of fusion | 32.0 kJ mol-1 |
| Heat of atomization | 659 kJ mol-1 |
| Heat of vaporization | 598 kJ mol-1 |
| 1st ionization energy | 684.9 kJ mol-1 |
| 2nd ionization energy | 1588.2 kJ mol-1 |
| 3rd ionization energy | 2620.5 kJ mol-1 |
| Electron affinity | 72 kJ mol-1 |
| Minimum oxidation number | -2 |
| Min. common oxidation no. | 0 |
| Maximum oxidation number | 6 |
| Max. common oxidation no. | 6 |
| Electronegativity (Pauling Scale) | 1.66 |
| Polarizability volume | 12.8 Å3 |
| Reaction with air | w/ht, ⇒ MoO3 |
| Reaction with 15 M HNO3 | none |
| Reaction with 6 M HCl | none |
| Reaction with 6 M NaOH | – |
| Oxide(s) | MoO2 (brown), MoO3 (white) |
| Hydride(s) | – |
| Chloride(s) | MoCl2, MoCl3, MoCl4, MoCl5, MoCl6 |
| Atomic radius | 139 pm |
| Ionic radius (1+ ion) | – |
| Ionic radius (2+ ion) | – |
| Ionic radius (3+ ion) | 83 pm |
| Ionic radius (1- ion) | – |
| Ionic radius (2- ion) | – |
| Ionic radius (3- ion) | – |
| Thermal conductivity | 138 W m-1 K-1 |
| Electrical conductivity | 17.3 x 106 S m-1 |
| Freezing/Melting point: | 2623 oC, 2896 K |

Lumps of molybdenum. Photo by Tomihahndorf.
Discovery of Molybdenum
Molybdenite, also known as molybdena, is a soft black mineral that was once used to make pencils. The mineral was often confused for graphite and it was thought to contain lead. It is now known to be molybdenum disulfide (MoS2).
In 1778 Swedish scientist Carl W. Scheele proved that molybdenite was not graphite nor did it contain lead. Nitric acid does not react with graphite, while the molybdenite produced sulfuric acid and a white solid – we now know this was molybdenum oxide or possibly molybdenum oxide hydrate. (1)
Scheele concluded that the mineral contained a new element, but he did not isolate it, because he did not have a suitable furnace to reduce the white solid to the metal.
In 1781, Scheele’s friend and countryman, Peter J. Hjelm isolated the metal by reducing the white solid with carbon. He ground the two substances together using linseed oil to form a paste – the paste ensured intimate contact between the carbon and the molybdenite. Hjelm heated the mixture strongly in a closed crucible to produce the new metallic element. (2) Hjelm called his new metal molybdenum.
The element name comes from the Greek word ‘molybdos’ meaning lead.

Without Molybdenum, Nothing Could Live
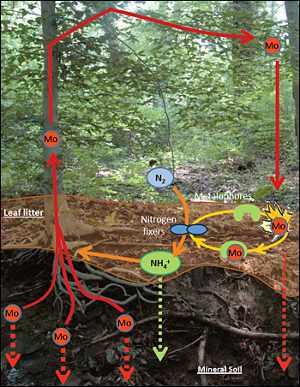
The molybdenum (Mo) cycle in soil: Molybdenum in the soil is taken up by tree roots and deposited in leaves. These leaves fall to the ground and decompose, releasing molybdenum. Image credit: Brookhaven National Laboratory
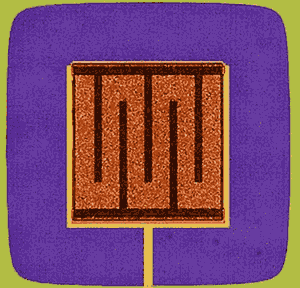
The NIST “transition-edge sensor” is made of layers of molybdenum and copper. It is used it X-ray sensors for materials research and astronomy. Image credit: NIST
Appearance and Characteristics
Harmful effects:
Molybdenum is toxic in all but small quantities.
Characteristics:
Molybdenum is a silvery-white, high-melting metal.
It does not react with oxygen or water at room temperature and it also resists corrosion at ordinary temperatures.
When present in compounds, molybdenum exists mostly in the oxidation state IV and VI.
Molybdenum is one of the five major refractory metals (metals with very high resistance to heat and wear).
The Five Refractory Metals – note their close relationship in the periodic table
The other refractory metals are tungsten, tantalum, rhenium and niobium.
Molybdenum oxide (MoO3) is soluble in alkaline water, forming molybdate salts.
Uses of Molybdenum
Molybdenum is used in small quantities to harden steel and is used in many alloys.
Molybdenum’s strength and resistance to expanding or softening at high temperatures is particularly sought after in critical areas where high temperatures are common, such as in nuclear power plants and aircraft engines.
Molybdenum is used as glass furnace electrodes due to its high melting point.
It is also used in the petroleum industry, to catalyze the removal of organic sulfur compounds in coal liquification and gas liquification processes.
Molybdenum is an essential trace element for animals and plants. As with selenium, too much of it is toxic, too little of it is fatal.
In nitrogen fixing bacteria, molybdenum is a vital component of the nitrogenase enzyme which allows conversion of nitrogen gas in air into nitrates vital for plant growth.
Molybdenum is also present in 20 or so enzymes needed in animals’ metabolisms.
Abundance and Isotopes
Abundance earth’s crust: 1.2 parts per million by weight, 0.2 parts per million by moles
Abundance solar system: 9 parts per billion by weight, 0.1 part per billion by moles
Cost, pure: $44 per 100g
Cost, bulk: $ per 100g
Source: Molybdenum metal is not found free in nature. The main ore of molybdenum is molybdenite, (molybdenum disulfide, MoS2). It also occurs in wulfenite (lead molybdate) and powellite (calcium molybdate). Commercially, the metal is obtained by mining molybdenite directly and it is also recovered as a by-product of copper mining.
Isotopes: Molybdenum has 24 isotopes whose half-lives are known with mass numbers from 86 to 110. Naturally occurring molybdenum is a mixture of seven isotopes and they are found in the percentages shown: 92Mo (14.8%), 94Mo (9.2%), 95Mo (15.9%), 96Mo (16.7%), 97Mo (9.6%), 98Mo (24.1%) and 100Mo (9.6%). The most naturally abundant is 98Mo at 24.1%.

References
- Mary Eagleson, Concise Encyclopedia Chemistry., Walter de Gruyter., 1994., p662.
- Mary Elvira Weeks, The Discovery of the Elements V., Journal of Chemical Education., March 1932., p 462.
Cite this Page
For online linking, please copy and paste one of the following:
or
To cite this page in an academic document, please use the following MLA compliant citation:
"Molybdenum." Chemicool Periodic Table. Chemicool.com. 17 Oct. 2012. Web.
https://www.chemicool.com/elements/molybdenum.html.