An armoured marine reptile from the Early Triassic of South China and its phylogenetic and evolutionary implications (original) (raw)
Abstract
Sauropterygia was a taxonomically and ecomorphologically diverse clade of Mesozoic marine reptiles spanning the Early Triassic to the Late Cretaceous. Sauropterygians are traditionally divided into two groups representing two markedly different body plans – the short-necked, durophagous Placodontia and the long-necked Eosauropterygia – whereas Saurosphargidae, a small clade of armoured marine reptiles, is generally considered as the sauropterygian sister-group. However, the early evolutionary history of sauropterygians and their phylogenetic relationships with other groups within Diapsida are still incompletely understood. Here, we report a new saurosphargid from the Early Triassic (Olenekian) of South China – Prosaurosphargis yingzishanensis gen. et sp. nov. – representing the earliest known occurrence of the clade. An updated phylogenetic analysis focussing on the interrelationships among diapsid reptiles recovers saurosphargids as nested within sauropterygians, forming a clade with eosauropterygians to the exclusion of placodonts. Furthermore, a clade comprising Eusaurosphargis and Palatodonta is recovered as the sauropterygian sister-group within Sauropterygomorpha tax. nov. The phylogenetic position of several Early and Middle Triassic sauropterygians of previously uncertain phylogenetic affinity, such as Atopodentatus, Hanosaurus, Majiashanosaurus, and Corosaurus, is also clarified, elucidating the early evolutionary assembly of the sauropterygian body plan. Finally, our phylogenetic analysis supports the placement of Testudines and Archosauromorpha within Archelosauria, a result strongly corroborated by molecular data, but only recently recovered in a phylogenetic analysis using a morphology-only dataset. Our study provides evidence for the rapid diversification of sauropterygians in the aftermath of the Permo-Triassic mass extinction event and emphasises the importance of broad taxonomic sampling in reconstructing phylogenetic relationships among extinct taxa.
Research organism: Other
eLife digest
Around 252 million years ago, just before the start of a period of time known as the Triassic, over 90% of animals, plants and other species on Earth went extinct in what was the worst mass extinction event in the planet’s history. It is thought to have happened because of an increase in volcanic eruptions that led to global warming, acid rain and other catastrophic changes in the environment.
The loss of so many species caused ecosystems to restructure as the surviving species evolved to fill niches left by those that had gone extinct. On land, reptiles diversified to give rise to dinosaurs, the flying pterosaurs, and the ancestors of modern crocodiles, lizards, snakes and turtles. Some of these land-based animals evolved to live in water, resulting in many species of marine reptiles emerging during the Triassic period.
This included the saurosphargids, a group of marine reptiles that lived in the Middle Triassic around 247–237 million years ago. They were ‘armoured’ with a shield made of broadened ribs superficially similar to that of turtles, and a covering of bony plates**.** However, it is unclear how the saurosphargids evolved and how closely they are related to other marine reptiles.
Here, Wolniewicz et al. studied a new species of saurosphargid named Prosaurosphargis yingzishanensis that was found fossilized in a quarry in South China. The animal was around 1.5 metres long and had a chest shield and armoured plates like other saurosphargids. The characteristics of the rock surrounding the fossil suggest that this individual lived in the Early Triassic, several million years before other saurosphargid species.
The team used a phylogenetic approach to infer the evolutionary relationships between P. yingzishanensis and numerous other land-based and marine reptiles based on over 220 anatomical characteristics of the animals. The resulting evolutionary tree indicated that the saurosphargids represented an early stage in the evolution of a larger group of marine reptiles known as the sauropterygians. The analysis also identified the closest land-based relatives of sauropterygians.
These findings provide evidence that marine reptiles rapidly diversified in the aftermath of the mass extinction event 252 million years ago. Furthermore, they contribute to our understanding of how ecosystems recover after a major environmental crisis.
Introduction
Several groups of reptiles invaded the marine realm in the aftermath of the Permo-Triassic mass extinction (PTME), the largest extinction event in Earth’s history (Benton, 2015). This phenomenon was likely a result of the scarcity of marine competitors and predators caused by the PTME and high productivity in the incipient shallow marine environment (Vermeij and Motani, 2018). Triassic marine reptiles, including the iconic Ichthyosauromorpha and Sauropterygia, as well as some other smaller and lesser known groups, achieved high taxonomic and ecological diversity rapidly after their emergence in the late Early Triassic and played a pivotal role in the reorganisation of marine food webs following the PTME (Scheyer et al., 2014; Kelley and Pyenson, 2015; Motani et al., 2015; Motani et al., 2017; Jiang et al., 2016; Stubbs and Benton, 2016; Cheng et al., 2019; Moon and Stubbs, 2020; Li and Liu, 2020; Sander et al., 2021; Qiao et al., 2022). Because Mesozoic marine reptiles represent likely several independent transitions from a terrestrial to an aquatic lifestyle, they also provide an ideal system to analyse the roles of function and constraint in determining evolutionary pathways (Motani, 2009; Benson, 2013).
Sauropterygia was a diverse clade of Mesozoic marine reptiles that first appeared in the late Early Triassic (Jiang et al., 2014; Li and Liu, 2020) and its members remained important predators in marine ecosystems until their extinction at the end of the Late Cretaceous (Bardet, 1994; Rieppel, 2000a; Benson and Druckenmiller, 2014). Sauropterygia is traditionally divided into two major lineages, representing two markedly different body plans – the Placodontia and the Eosauropterygia, the latter comprising Pachypleurosauria, Nothosauroidae and Pistosauroidea (which includes the iconic Plesiosauria; Rieppel, 2000a). Placodonts were characterised by the presence of short necks and short skulls and possessed crushing palatal dentition almost certainly used for feeding on hard-shelled invertebrates (Rieppel, 2002; Crofts et al., 2017). Some early-diverging placodonts had a limited covering of osteoderms on their backs and possibly limbs (Jiang et al., 2008; Klein and Scheyer, 2013), but derived forms evolved extensive dorsal armour superficially similar to that of turtles (Scheyer, 2007; Wang et al., 2020). Eosauropterygians, on the other hand, had elongated necks and elongated skulls with pointed dentition suitable for capturing fast-moving prey (Rieppel, 2000a; Rieppel, 2002). Placodonts remained restricted to shallow marine environments until their extinction in the Late Triassic, whereas eosauropterygians evolved a suite of adaptations for a pelagic lifestyle and became one of the dominant groups of marine reptiles in the Jurassic and Cretaceous (Kelley et al., 2014).
Even though sauropterygians have a rich fossil record and a long history of scientific research, the early evolution of the group is still incompletely understood. In a broad phylogenetic context, sauropterygians have been consistently recovered within Diapsida, but their exact phylogenetic position relative to other diapsid groups remains unresolved. Several diapsid clades, including Saurosphargidae (Li et al., 2014; Li et al., 2018; Neenan et al., 2015; Wang et al., 2022), Testudines (deBraga and Rieppel, 1997; Schoch and Sues, 2015), Ichthyosauromorpha (Martínez et al., 2021), Thalattosauria (Simões et al., 2022) and the armoured reptile Eusaurosphargis dalsassoi (Scheyer et al., 2017) were previously proposed to be the sauropterygian sister-group, but its exact identity is still a matter of debate. Because sauropterygians were suggested as being closely related not only to turtles, but also to archosauromorphs (Chen et al., 2014a; Neenan et al., 2015; Martínez et al., 2021; Simões et al., 2022), resolving their phylogenetic placement within diapsids is of crucial importance for solving the phylogenetic uncertainty surrounding Archelosauria – a clade comprising turtles and archosauromorphs strongly supported by molecular data (Crawford et al., 2015; Lyson and Bever, 2020), but only recently recovered in a phylogenetic analysis using a morphology-only dataset (Simões et al., 2022).
Saurosphargids are a small clade of Mesozoic marine reptiles characterised by the presence of body armour comprising broadened dorsal ribs, forming a closed ‘rib-basket’, and a moderately- to well-developed osteoderm covering (Li et al., 2011; Li et al., 2014). Saurosphargids are known from the Middle Triassic of Europe and South China, although recent evidence suggests they could have survived as late as the Late Triassic (Scheyer et al., 2022). Saurosphargids comprise as many as four taxa – Saurosphargis volzi (considered a nomen dubium by some authors) (Huene, 1936; Nosotti and Rieppel, 2003; Scheyer et al., 2017), the heavily armoured Sinosaurosphargis yunguiensis (Li et al., 2011; Hirasawa et al., 2013), and two species in the genus Largocephalosaurus (L. polycarpon and L. qianensis; Cheng et al., 2012; Li et al., 2014). Saurosphargids are one of the reptile groups proposed as the sister-group of sauropterygians (see above), but some recent phylogenetic analyses have suggested their placement within sauropterygians instead, as either the sister-group to placodonts (Schoch and Sues, 2015; Simões et al., 2018; Martínez et al., 2021) or eosauropterygians (Scheyer et al., 2017; Wang et al., 2019; Simões et al., 2022).
Our understanding of the early evolution of the sauropterygian body plan is also hindered by the uncertain phylogenetic position of several Early and Middle Triassic taxa. Hanosaurus hupehensis (Young, 1972; Rieppel, 1998a; Wang et al., 2022) and Majiashanosaurus discocoracoidis (Jiang et al., 2014) from the Early Triassic of South China are variably recovered as either lying outside of the clade comprising Saurosphargidae + Sauropterygia (Hanosaurus; Wang et al., 2022), as pachypleurosaurs (Jiang et al., 2014; Neenan et al., 2015; Lin et al., 2021; Wang et al., 2022), or as outgroups to a clade comprising nothosauroids and pachypleurosaurs to the exclusion of pistosauroids (Li and Liu, 2020). The phylogenetic placement of Corosaurus alcovensis from the Early–Middle Triassic of Wyoming, USA, is also unresolved, with different authors arguing for its early-diverging eosauropterygian (Rieppel, 1994; Li and Liu, 2020), eusauropterygian (more closely related to nothosauroids and pistosauroids than to pachypleurosaurs; Neenan et al., 2015; Lin et al., 2021), or pistosauroid (Rieppel, 1998b; Wang et al., 2022) affinity. The phylogenetic position of the herbivorous hammer-headed sauropterygian Atopodentatus unicus from the Middle Triassic of South China relative to placodonts and eosauropterygians also remains uncertain (Cheng et al., 2014; Li et al., 2016; Wang et al., 2022). These conflicting phylogenetic placements are likely the result of inadequate sampling of Early and Middle Triassic sauropterygian ingroup taxa, as well as the inclusion of only a limited number of diapsid reptile clades, including potential sauropterygian outgroups, in previous phylogenetic analyses.
Here, we report an Early Triassic saurosphargid from South China, representing the earliest recorded occurence of the group. We also present an updated phylogenetic hypothesis for Diapsida with a particular focus on Sauropterygia, which we use as context for discussing the early evolutionary assembly of the sauropterygian body plan.
Results
Geological background
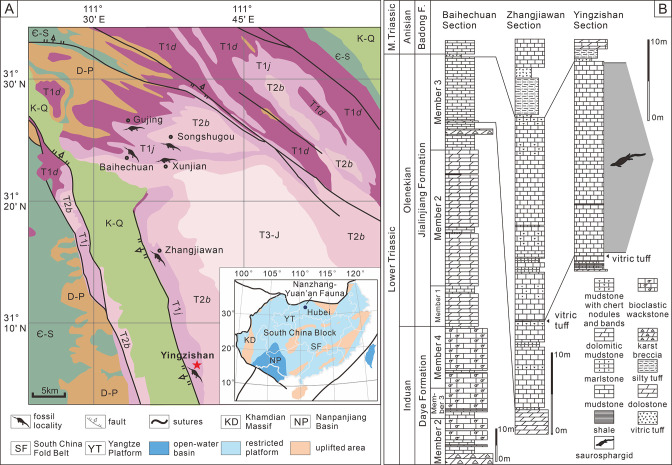
Yingzishan quarry, where the new specimen (HFUT YZSB-19-109) was collected, is located on the northern boundary of the Yangtze Platform (Figure 1A; Li and Liu, 2020). The new specimen represents the Early Triassic Nanzhang-Yuan’an fauna (Li and Liu, 2020) and originates from the upper part of the third member of the Jialingjiang Formation (Figure 1B). Traditionally, the Jialingjiang Formation has been divided into four members by most authors studying the region (Li et al., 2002; Zhao et al., 2008; Cheng et al., 2015). From base to top, the first member consists of thick-bedded to massive dolostone, the second member consists of vermicular limestone intercalated with dolostone, the third member consists of laminated thin- to medium-bedded limestone to dolostone, whereas the fourth member is composed of dolostone and karstified breccia. Thus, in the four-member division of the Jialingjiang Formation, the thick volcanic ash (Figure 1B) marks the bottom of the fourth member. However, recent geological mapping in the region (Chen et al., 2016a) proposed that the fourth member of the Jialingjiang Formation should be included in the Middle Triassic Badong Formation, indicating a three-member division of the Jialingjiang Formation. This division is consistent with the lithology of the Jianlingjiang and Badong formations as defined by the official geological guide of Hubei Province (Bureau of Geology and Mineral Resources of Hubei Province, 1990). The division of the Lower and Middle Triassic in the region is also consistent with the widespread thick volcanic ash as a marker of the Lower–Middle Triassic boundary in the Yangtze Platform (Chen et al., 2020). Consequently, this three-member division was followed by Cheng et al., 2019, Li and Liu, 2020, Qiao et al., 2019 and is also followed in this study.
Figure 1. Locality and horizon of Prosaurosphargis yingzishanensis (HFUT YZSB-19-109).
(A) The geological map of the Nanzhang-Yuan’an region showing Yingzishan quarry, where HFUT YZSB-19-109 was collected; inset is a paleogeographic map of the South China Block in the Triassic showing the location of the Nanzhang-Yuan’an fauna (after Qiao et al., 2019). (B) Stratigraphic column showing the horizon from which HFUT YZSB-19-109 was collected. Abbreviations: Є-S, Cambrian–Silurian; D-P, Devonian–Permian; F., Formation; K-Q, Cretaceous–Quaternary; M., Middle; T1_d_, Daye Formation, Lower Triassic; T1_j_, Jialingjiang Formation, Lower Triassic; T2_b_, Badong Formation, Middle Triassic; T3-J, Upper Triassic–Jurassic.
However, the second and third members of the Jialingjiang Formation were recently redefined by Yan et al., 2021, who regarded the lowermost base of the thick vitric tuff as the boundary between the second and third members (contra Chen et al., 2016a; Cheng et al., 2019). As a consequence, the Nanzhang-Yuan’an fauna was placed into the newly defined second member of the Jialingjiang Formation (Cheng et al., 2022; Zhao et al., 2022). We argue that this new definition and division of different members of the Jialingjiang Formation contradicts the official geological guide (Bureau of Geology and Mineral Resources of Hubei Province, 1990) and causes confusion. Therefore, we prefer to maintain the definition of the thick vitric tuff as the boundary between the Early Triassic Jialingjiang and the Middle Triassic Badong formations in the Nanzhang-Yuan’an region (Figure 1B), pending future updates of the official geological guide of Hubei Province.
The new specimen is buried in dark grey, laminated, and thin-bedded carbonate mudstone (Figure 1B) with some carbonaceous interactions. There are also some peloids, replacive dolomites, and microbial mats in the fossiliferous levels (Chen et al., 2022). Based on the published sedimentological accounts (Wang et al., 2011; Chen et al., 2016b; Yan et al., 2018; Yan et al., 2021; Li et al., 2020; Zhao et al., 2022) and field observations, a restricted, stagnant, and hypersaline lagoon within a tidal flat environment is inferred as the burial setting of the marine reptiles of the Nanzhang-Yuan’an fauna (Chen et al., 2022).
Systematic palaeontology
- Reptilia Laurenti, 1768
- Diapsida Osborn, 1903
- Archelosauria Crawford et al., 2015
- Sauropterygomorpha tax. nov.
Definition: The most recent common ancestor of Eusaurosphargis dalsassoi and Pistosaurus longaevus, and all of its descendants (min ∇ Eusaurosphargis dalsassoi Nosotti and Rieppel, 2003 & Pistosaurus longaevus Meyer, 1839).
Diagnosis: Osteoderms present (ch. 143.1), body strongly flattenned dorso-ventrally (ch. 144.1), clavicle applied to the medial surface of scapula (ch. 148.1), metatarsal V long and slender (ch. 186.0), metatarsal I less than 50% the length of metatarsal IV (ch. 189.1).
- Sauropterygia Owen, 1860
- Saurosphargidae Li et al., 2011
- Prosaurosphargis yingzishanensis gen. et sp. nov.
- urn:lsid:zoobank.org:act:36BED757-A86D-4951-B664-A91969F7CDBF urn:lsid:zoobank.org:act:825E86DD-E636-4A99-AA85-7AF4DBCD7E8B
Holotype: HFUT YZSB-19-109, a partial postcranial skeleton (Figure 2). The specimen is housed in the Geological Museum of Hefei University of Technology, Hefei, Anhui Province, China (HFUT).
Figure 2. Holotype of Prosaurosphargis yingzishanensis (HFUT YZSB-19-109) (A) and skeletal reconstruction with known elements highlighted in white (gastralia removed for clarity) (B).
Etymology: Genus name from the Greek preposition πρό (pró), meaning before, and Saurosphargis, the name of the type genus of the family Saurosphargidae (Li et al., 2011). The specific epithet refers to the type locality (see above).
Horizon and locality: Third Member of the Jialingjiang Formation (uppermost Spathian, Olenekian, Lower Triassic), Yingzishan quarry, Yuan’an County, Hubei Province, China.
Diagnosis: A saurosphargid characterised by the following combination of character states: (1) spaces between dorsal transverse processes anteroposteriorly shorter than the anteroposterior widths of the transverse processes (like in Saurosphargis and Sinosaurosphargis, but different from Largocephalosaurus, in which the spaces between the dorsal transverse processes are wider than the transverse processes); (2) ribs without uncinate processes (like in Sinosaurosphargis, but unlike in Saurosphargis and Largocephalosaurus, in which uncinate processes are present); (3) osteoderms forming a median, parasaggital and lateral rows (similar to Largocephalosaurus polycarpon, but different from L. qianensis, in which additional, small osteoderms more extensively cover the lateral sides of the body, and different from Sinosaurosphargis, in which the osteoderms form an extensive dorsal armour); (4) ectepicondylar groove on humerus present (like in Largocephalosaurus qianensis, absent in L. polycarpon); (5) entepicondylar foramen in humerus absent (as in Largocephalosaurus) (details of humerus morphology unknown in Sinosaurosphargis); (6) radius short relative to humerus compared with other saurosphargids; (7) presence of a single distal tarsal (distal tarsal IV) (different from Largocephalosaurus, which possesses two distal tarsals – III and IV) (number of tarsals unknown in Sinosaurosphargis); (8) anterior caudal ribs shorter than sacral ribs (anterior caudal ribs longer than sacral ribs in Largocephalosaurus, unknown in Sinosaurosphargis).
Description and comparisons
HFUT YZSB-19-109 comprises three blocks (Figure 2). The first large block contains mostly disarticulated parts from the anterior right portion of the postcranial skeleton – 2 cervical neural arches and 5 cervical ribs, 1 dorsal centrum and 2 dorsal neural arches (all three preserved in articulation), 9 dorsal ribs, 1 median, 11 lateral and 13 lateralmost gastral elements, a single parasaggital osteoderm, the right scapula, right coracoid, right humerus, and right radius (Figure 3). The second small block contains the distal ends of the six most anterior dorsal ribs, five lateralmost gastral elements, as many as eight osteoderms, a partial left coracoid, and a left humerus (Figure 4). The third large block preserves the articulated posterior part of the body, including 5 posterior dorsal neural arches and associated ribs, 4 posterior dorsal/sacral vertebrae and ribs, an articulated series of 11 anterior caudals with associated ribs, chevrons and osteoderms, 14 lateralmost gastralia, a single parasaggital osteoderm, a partial right and a complete left pelvic girdle, and a complete left hindlimb (Figures 5 and 6). Based on the humerus:total body length and femur:total body length ratios of the type specimen of Largocephalosaurus qianensis (specimen IVPP V 15638, total body length = 2317 mm; Li et al., 2014), the only completely preserved saurosphargid specimen discovered to date, the total length of HFUT YZSB-19-109 is estimated to have reached between 1468 mm to 1583 mm, respectively. Selected measurements of HFUT YZSB-19-109 are given in Table 1.
Figure 3. Anterior vertebral column, right pectoral girdle, and right forelimb elements of Prosaurosphargis yingzishanensis.
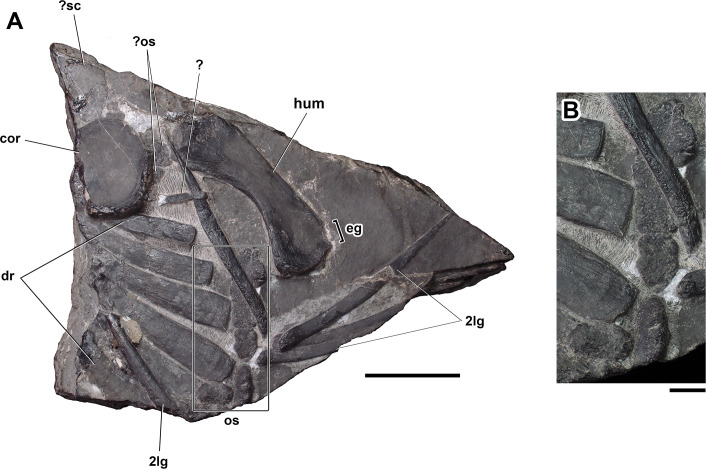
Abbreviations: 1lg, first lateral gastral element; 2lg, second lateral gastral element; cna, cervical neural arch; cor, coracoid; cr, cervical rib; dc, dorsal centrum; dna, dorsal neural arch; dr, dorsal rib; hum, humerus; mg, median gastral element; os, osteoderm; rad, radius; sc, scapula. Scale bar = 5 cm.
Figure 4. Dorsal ribs, osteoderms, coracoid, and humerus from the left side of the body of Prosaurosphargis yingzishanensis (A) and detail of lateral osteoderms (B).
Abbreviations: 2lg, second lateral gastral element; cor, coracoid; dr, dorsal rib; eg, ectepicondylar groove; hum, humerus; os, osteoderm, ?sc, ?scapula. Scale bar = 5 cm in (A) and 1 cm in (B).
Figure 5. Pelvic girdle and hindlimb of Prosaurosphargis yingzishanensis.
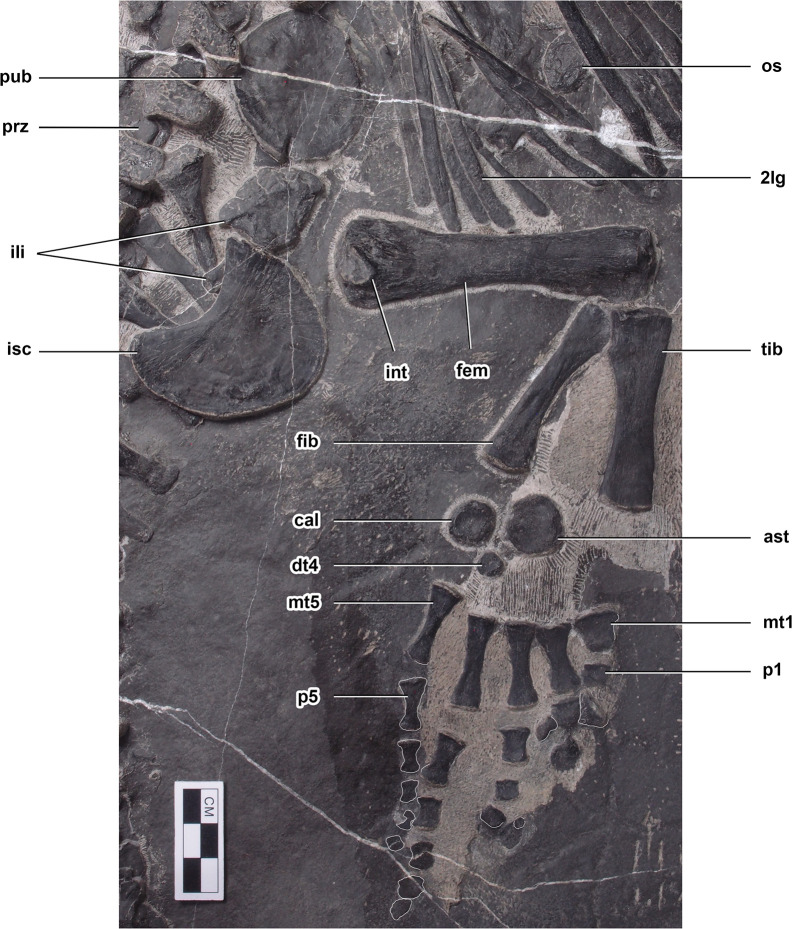
Abbreviations: 2lg, second lateral gastral element; ast, astragalus; cal, calcaneum; dt4, distal tarsal IV; fem, femur; fib, fibula; ili, ilium; int, internal trochanter; isc, ischium; mt1, metatarsal I; mt5, metatarsal V; os, osteoderm; p1, first phalanx of digit 1; p5, first phalanx of digit 5; pub, pubis; prz, prezygapophysis; tib, tibia. Scale bar = 3 cm.
Figure 6. Sacral and caudal vertebrae of Prosaurosphargis yingzishanensis (A), close-up of anterior caudal vertebrae (B), and close-up of more posterior caudal vertebrae (C).
Abbreviations: ch, chevron; cr2, second caudal rib; cr3, third caudal rib; crs6-7, sixth and seventh caudal ribs; dr, dorsal rib; ns, neural spine; os, osteoderm; prz, prezygapophysis; rf, rib facet; rgs, rugose surface; sr, sacral rib. Scale bar = 5 cm in (A) and 1 cm in (B) and (C).
Table 1. Selected measurements of HFUT YZSB-19-109, holotype of Prosaurosphargis yingzishanensis.
| Vertebral column | |
|---|---|
| Centrum position | Maximum anteroposterior length(measured along mid-dorsoventral height) |
| Last dorsal | 18.25 mm |
| 1st sacral | 19.45 mm |
| 2nd sacral | 20.49 mm |
| 3rd sacral | 20.50 mm |
| 1st caudal | 20.50 mm |
| 2nd caudal | 18.35 mm |
| 3rd caudal | 19.40 mm |
| 4th caudal | 20.14 mm |
| 5th caudal | 18.64 mm |
| 6th caudal | 19.10 mm |
| 7th caudal | 17.95 mm |
| 8th caudal | 18.84 mm |
| 9th caudal | 18.10 mm |
| 10th caudal | 17.86 mm |
| Pectoral girdle | |
| Right scapula | |
| Maximum proximodistal length | 68.09 mm |
| Maximum proximal width | 51.20 mm |
| Right coracoid | |
| Maximum proximodistal length | 51.65 mm |
| Left coracoid | |
| Maximum proximodistal length | 52.25 mm |
| Maximum anteroposterior length | 39.40 mm |
| Forelimb | |
| Right humerus | |
| Maximum proximodistal length | 104.44 mm |
| Maximum proximal width | 38.85 mm |
| Maximum distal width | 27.80 mm |
| Left humerus | |
| Maximum anteroposterior length | 104.24 mm |
| Minimum mediolateral width | 20.10 mm |
| Maximum distal width | 31.50 mm |
| Right radius | |
| Maximum proximodistal length(measured along proximodistal axis) | 58.50 mm |
| Maximum proximodistal length(measured along anterior margin) | 60.95 mm |
| Maximum proximodistal length(measured along posterior margin) | 51.10 mm |
| Pelvic girdle | |
| Left ilium | |
| Maximum proximodistal length | 23.20 mm |
| Maximum distal width | 32.99 mm |
| Left ischium | |
| Maximum anteroposterior (proximodistal) length | 51.44 mm |
| Maximum mediolateral width | 36.59 mm |
| Left pubis | |
| Maximum proximodistal length | 43.25 mm |
| Maximum anteroposterior length | 36.85 mm |
| Hindlimb | |
| Left femur | |
| Maximum proximodistal length | 93.85 mm |
| Maximum proximal width | 27.65 mm |
| Maximum distal width | 23.40 mm |
| Minimum mediolateral (anteroposterior) width | 14.25 mm |
| Left tibia | |
| Maximum proximodistal length | 60.35 mm |
| Maximum proximal width | 18.29 mm |
| Maximum distal width | 16.39 mm |
| Minimum mediolateral (anteroposterior) width | 10.90 mm |
| Left fibula | |
| Maximum proximodistal length | 55.59 mm |
| Maximum proximal width | 12.90 mm |
| Maximum distal width | 15.24 mm |
| Minimum mediolateral (anteroposterior) width | 7.25 mm |
| Astragalus | |
| Maximum proximodistal length | 18.45 mm |
| Maximum mediolateral (anteroposterior) width | 18.20 mm |
| Calcaneum | |
| Maximum proximodistal length | 14.25 mm |
| Maximum mediolateral (anteroposterior) width | 13.55 mm |
| Distal tarsal IV | |
| Maximum proximodistal length | 9.49 mm |
| Maximum mediolateral (anteroposterior) width | 8.95 mm |
| Metacarpals | |
| Metacarpal I maximum proximodistal length | 13.44 mm |
| Metacarpal II maximum proximodistal length | 20.89 mm |
| Metacarpal III maximum proximodistal length | 25.89 mm |
| Metacarpal IV maximum proximodistal length | 28.19 mm |
| Metacarpal V maximum proximodistal length | 24.25 mm |
Axial skeleton
Vertebrae: The vertebral column of HFUT YZSB-19-109 is represented by two disarticulated cervical neural arches, a single anterior dorsal centrum and two anterior dorsal neural arches (all three preserved in articulation), and an articulated series of 20 vertebrae comprising five posterior dorsal neural arches, four complete posterior dorsal/sacral vertebrae and 11 complete caudal vertebrae.
Two cervical neural arches are preserved in HFUT YZSB-19-109 (Figure 3). Only the left side of a small neural arch, representing one of the anterior post-axial cervicals, is preserved in dorsal view, whereas a larger neural arch, belonging to one of the posterior cervicals, is completely preserved and exposed in ventral view, but it is partially obscured by an overlying cervical rib and is rotated by approximately 180 degrees, so that its anterior end faces posteriorly. The transverse processes of the cervical neural arches extend posterolaterally and are proportionally shorter than the transverse processes of the dorsal neural arches. A well-developed prezygapophysis is clearly visible in the smaller neural arch. Well-developed postzygapophyses are preserved in both the anterior and posterior cervical neural arches, where they are visible protruding from just underneath the overlying cervical rib in the latter. Approximately oval, anteroposteriorly elongate facets for articulation with the centrum are exposed in the posterior cervical neural arch.
The only preserved dorsal centrum is exposed in ventral view (Figure 3). It is mediolaterally constricted at its anteroposterior mid-length, resembling the dorsal centra of other saurosphargids (Huene, 1936; Li et al., 2011; Li et al., 2014). The pedicels of the dorsal neural arches are anteroposteriorly elongate and mediolaterally narrow, resembling those preserved in Saurosphargis (Huene, 1936). The transverse processes are straight and mediolaterally elongate. The maximum width of the transverse process is greater than the maximum width of the space between the transverse processes of adjacent neural arches (Figure 3), a condition similar to that exhibited by Saurosphargis (Huene, 1936) and Sinosaurosphargis (Li et al., 2011), but different from Largocephalosaurus (Li et al., 2014) and Eusaurosphargis (Scheyer et al., 2017), in which the widths of the spaces between the dorsal transverse processes are approximately equal or greater than the widths of the transverse processes.
Five posterior dorsal neural arches are preserved in articulation in HFUT YZSB-19-109 and are exposed in ventral view. In adddition, a fragmentary centrum is associated with the second preserved posterior dorsal neural arch. The articulation surfaces for the centra located on the pedicels of the posterior dorsal neural arches are anteroposteriorly elongate, but seem mediolaterally broader and not as well demarcated from the transverse processes as those in the more anterior dorsal neural arches. The posterior dorsal transverse processes gradually become mediolaterally shorter and anteroposteriorly broader posteriorly. Well-developed prezygapophysis-postzygapophysis articulations are preserved and exposed between the second and fifth posterior dorsal neural arches. Articulated anterior and posterior dorsal neural arches preserved without their respective centra indicate a lack of fusion of the neurocentral suture, a paedomorphic feature characteristic for Sauropterygia (Rieppel, 2000a) and also present in Saurosphargis (Huene, 1936) and Largocephalosaurus (Li et al., 2014).
Four complete posterior dorsal/sacral vertebrae are preserved and exposed in left ventrolateral view. Two sacral vertebrae can be identified by the presence of associated sacral ribs with clearly expanded distal ends. However, the distal ends of the first two ribs lying immediately posterior to the last unambiguous dorsal rib are obscured by the overlying ilium and ischium, so it is not possible to confidently determine whether they represent the last two dorsal ribs or the first two sacral ribs (Figure 6A). A series of 11 articulated and complete caudal vertebrae is also preserved, with two anterior caudals exposed in left ventrolateral view and the remaining caudals exposed in lateral view (Figure 6). The sacral and caudal centra have concave lateral and ventral surfaces and are likely amphicoelus, as evidenced by the concave anterior articular surface of the second caudal centrum, but in most cases the articular surfaces are obscured by matrix or adjacent centra, giving a false impression of procoely or amphiplaty. The caudal centra are dorsoventrally taller than anteroposteriorly long and gradually decrease in size posteriorly. The caudal neural arches bear well-developed, anterodorsally inclined prezygapophyses. The neural spines of some caudal neural arches are exposed in lateral view (Figure 6B). They are dorsoventrally short, anteroposteriorly broad and possess a convex dorsal margin. A slightly rugose/striated dorsal surface, reminiscent of a similar surface present in the dorsal neural spines of Helveticosaurus (Rieppel, 1989a), Augustasaurus (Sander et al., 1997), Nothosaurus (Klein et al., 2022), and Pomolispondylus (Cheng et al., 2022), is preserved in some of the anterior caudal neural spines. Rib facets are visible from the second sacral to the penultimate preserved caudal vertebra (Figure 6). The dorsal portions of the rib facets are located on the ventrolateral surfaces of the neural arches, whereas their ventral portions are located on the dorsolateral surfaces of the centra and are demarcated by a prominent, ventrally arcuate ridge (Figure 6B). The surface of the rib facets is rugose. The last visible minute caudal rib is preserved in articulation with the seventh caudal vertebra, but small rib facets are also preserved in the following three vertebrae (Figure 6C). It is not clear, however, if small ossified caudal ribs were associated with these vertebrae in life.
Ribs: Six cervical ribs are preserved in HFUT YZSB-19-109 (Figures 3 and 4). Three anterior cervical ribs are approximately straight and possess a single, expanded head and narrow distal ends. Two posterior cervical ribs are also preserved, but their proximal portions are obscured by the overlying coracoid and scapula. These ribs are much longer than the anterior cervical ribs and possess slightly curved shafts and relatively broad distal ends. HFUT YZSB-19-109 also preserves 16 dorsal ribs, but most of them are incomplete and/or obscured by overlying skeletal elements, such as gastralia or other ribs (Figures 3, 4 and 6A). The anterior dorsal ribs are much more robust than the posterior cervical ribs, being proximodistally longer and anteroposteriorly much broader (Figures 3 and 4). They have a single head and greatly expanded distal portions that abut against each other, forming a characteristic ‘rib-basket’ also present in other saurosphargids (Huene, 1936; Li et al., 2011; Li et al., 2014), but differ from the dorsal ribs of Eusaurosphargis, which are narrow and widely spaced (Scheyer et al., 2017). The dorsal ribs do not bear a distinct uncinate process, being similar in this respect to the dorsal ribs of Sinosaurosphargis (Li et al., 2011), but differ from the dorsal ribs of Largocephalosaurus, Saurosphargis, Eusaurosphargis and a possible isolated saurosphargid rib from the Late Triassic of Switzerland, all of which possess uncinate processes (Li et al., 2014; Scheyer et al., 2017; Scheyer et al., 2022).
Five to seven posterior dorsal ribs are preserved in HFUT YZSB-19-109; they have an expanded head, a narrow distal end and are much shorter and slender in comparison with the more anterior dorsal ribs. As a consequence, they did not form part of the closed ‘rib-basket’. Two to four pairs of sacral ribs are preserved in HFUT YZSB-19-109, although the right sacral ribs are damaged and partially preserved as impressions (see above regarding the uncertainty in establishing the correct number of sacral vertebrae/rib pairs; Figure 6A). Two unambiguous sacral ribs are proximodistally slightly longer than the last evident dorsal rib and possess clearly expanded distal ends. The penultimate left sacral rib bears a small posteroproximal process. The caudal ribs are proximodistally short, possess a broad head and taper distally (Figure 6). They are not fused with the caudal centra. The anterior caudal ribs in HFUT YZSB-19-109 are shorter than the sacral ribs, in contrast to Largocephalosaurus, in which the anterior caudal ribs are longer than the sacral ribs (Li et al., 2014). The caudal ribs decrease in size posteriorly and extend at least to the level of the seventh caudal centrum, although it is not clear if caudal ribs extended posteriorly beyond the seventh caudal (see above). In Largocephalosaurus, the caudal ribs extend to the level of the 10th or 11th caudal centrum (Li et al., 2014).
Chevrons: Eight chevrons are preserved in HFUT YZSB-19-109 in association with some of the posterior caudal vertebrae (Figure 6A and C). In lateral view, the chevrons are slender and straight or display a gentle ventral curvature. The chevrons have a proximal end that is approximately equal in size or only slightly expanded relative to the distal end.
Gastralia: The gastral rib basket is partially preserved in HFUT YZSB-19-109 (Figures 3—5). Like in Eusaurosphargis, Placodus and eosauropterygians (Drevermann, 1933; Rieppel, 1989b; Nosotti and Rieppel, 2003; Shang et al., 2011), each gastral rib was composed of five elements – a median element, two lateral (first lateral) elements and two lateralmost (second lateral) elements. Gastral ribs comprising five elements were also inferred for Saurosphargis (Huene, 1936), whereas in Sinosaurosphargis the gastral ribs were described as comprising three elements – one median and two lateral elements (Li et al., 2011), and the number of gastral rib elements was not specified for Largocephalosaurus (Li et al., 2014). A single anterior median gastral element, 11 lateral and 33 lateralmost elements are preserved in HFUT YZSB-19-109. The median element is weakly angulated anteriorly, whereas the first lateral elements form proportionally short rods with a blunt medial end and a pointed lateral end (Figure 3) and closely resemble the first lateral gastral elements of Placodus (Drevermann, 1933). The lateralmost elements are concentrated into two articulated series comprising 13 (anterior block; Figure 3) and 14 (posterior block; Figure 5) elements each. They form mediolaterally elongate rods which are approximately straight or gently angulated posteriorly. The medial end of the second lateral element is narrow and tapers into a pointed apex, whereas the lateral end is broadened and blunt with a distinctly rugose/granulated surface. One anteriorly positioned first lateral element seems to bifurcate laterally, forming two lateral prongs (Figure 3).
Osteoderms
Several osteoderms are preserved in HFUT YZSB-19-109. A single osteoderm is preserved in the anterior block between the anterior dorsal ribs (Figure 3) and a second isolated osteoderm is preserved in the posterior block between the posterior gastral elements (Figure 5). In addition, two possible osteoderms are also preserved in association with the proximal end of the left humerus (Figure 4). These osteoderms are approximately oval in outline and likely represent osteoderms forming parasaggital rows similar to those in Largocephalosaurus (Li et al., 2014; Scheyer et al., 2022). A partial series comprising six osteoderms is preserved along the distal ends of a series of partially preserved left dorsal ribs (Figure 4). These osteoderms represent the left lateral osteoderm row and are anteroposteriorly elongate, have an irregular, sub-oval, or sub-rectangular outline, and a densely pitted surface. One of the posterior elements in this series bears a prominent ridge extending along the midline of its exposed surface. In all these features, these osteoderms closely resemble the lateral osteoderms reported for Saurosphargis (Huene, 1936) and Largocephalosaurus polycarpon (Li et al., 2014).
Another partial series of small osteoderms is preserved lateral to the distal ends of the right sacral and anterior caudal ribs, indicating that the lateral osteoderm rows extended to the level of the seventh caudal vertebra (Figure 6A). A few small osteoderms are also preserved in close association with the anterior caudal neural spines and seem to have formed a median row along the dorsal midline (Figure 6B), likely overlying the caudal neural spines in a manner similar to that in Largocephalosaurus (Li et al., 2014) and Placodus (Jiang et al., 2008). In L. qianensis, a dense covering of small osteoderms is present on the dorsal surface of the neck, trunk and caudal region (Li et al., 2014), but no such osteoderms were found in association with HFUT YSZB-19-109. A dense covering of osteoderms is also absent in L. polycarpon (Cheng et al., 2012; Li et al., 2014).
Pectoral girdle
Scapula: The right scapula is completely preserved in HFUT YZSB-19-109 (Figure 3). In addition, a large and broad broken bone fragment likely representing the left scapula is visible lying anterodorsally to the left coracoid (Figure 4). The scapula possesses an anteroproximally expanded glenoid portion and a much narrower, straight, and posterodorsally projecting scapular blade. The glenoid portion forms a low acromion process proximodorsally. The coracoid facet is relatively broad and convex, whereas the glenoid facet is straight and short and oriented nearly parallel to the long axis of the scapular blade. The scapular blade is separated from the glenoid portion by a deep posteroventral notch. The scapular blade is straight, with gently concave anterodorsal and posteroventral margins and an approximately straight posterior margin. In general shape and proportions, the scapula of HFUT YZSB-19-109 closely resembles the scapula of Largocephalosaurus (Cheng et al., 2012; Li et al., 2014) and Corosaurus (Storrs, 1991), although the notch separating the glenoid portion from the scapular blade is much deeper and narrower in the latter.
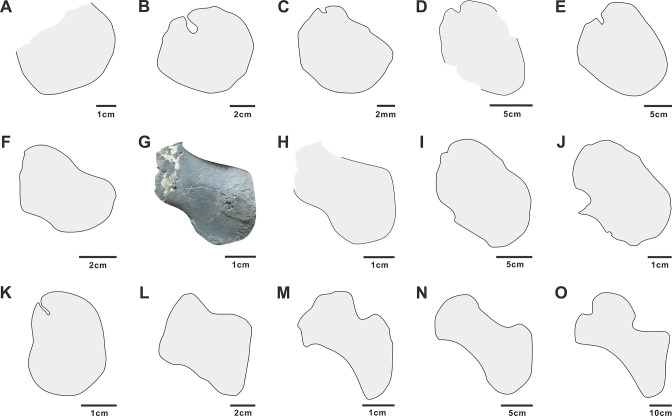
Coracoid: The coracoids are only partially visible in HFUT YZSB-19-109 – the right coracoid is almost entirely covered by the right scapula (Figure 3), whereas the proximal portion of the left coracoid is broken (Figure 4). The preserved parts of both coracoids indicate that it was a dorsoventrally flat, plate-like element, approximately sub-circular in outline, closely resembling the coracoid of Largocephalosaurs (Li et al., 2014; Figure 7). The coracoid of HFUT YZSB-19-109 differs, however, from the coracoids of Helveticosaurus (Rieppel 1989), Eusaurosphargis, Atopodentatus, early diverging placodonts, Majiashanosaurus, a referred specimen of Hanosaurus, and Lariosaurus sanxiaensis, in which the coracoid is proximodistally more elongate and approximately sub-oval in outline (Figure 7). It also differs from the coracoids of eosauropterygians, which possess weakly- or well-developed anterior and posterior emarginations (Figure 7). A small notch in the proximal part of the right coracoid, seen just above the anterior margin of the right scapula, likely represents the coracoid foramen. The exposed ventral surface of the left coracoid bears numerous radial striations extending from the centre of the bone towards its outer margins.
Figure 7. Coracoids of selected Early and Middle Triassic sauropterygians.
A - Prosaurosphargis (based on specimen HFUT YZSB-19-109); B - Largocephalosaurus (after Li et al., 2014); C - Eusaurosphargis (after Scheyer et al., 2017); D - Atopodentatus (after Cheng et al., 2014); E - Placodus (after Drevermann, 1933); F - Paraplacodus (after Rieppel, 2000b); G (photo) and H (outline) - Hanosaurus (holotype, based on specimen IVPP V 3231); I - Hanosaurus (referred specimen; after Wang et al., 2022); J - Lariosaurus sanxiaensis (after Li and Liu, 2020); K – Majiashanosaurus (after Jiang et al., 2014), L – Corosaurus (after Storrs, 1991); M - Wumengosaurus (after Wu et al., 2011); N – Anarosaurus (after Klein, 2012); O – Nothosaurus (after Ji et al., 2014).
Forelimb
Humerus: Both humeri are preserved in HFUT YZSB-19-109 – the right humerus is complete, but partially overlapped by the right scapular blade (Figure 3), whereas the left humerus is slightly broken proximally (Figure 4). The humerus is proximodistally elongate; it is also posteriorly curved along its proximodistal axis, like the humerus of other saurosphargids (Li et al., 2011; Li et al., 2014; Cheng et al., 2012), Placodus (Jiang et al., 2008), Majiashanosaurus (Jiang et al., 2014), Lariosaurus sanxiaensis (Li and Liu, 2020), a referred specimen of Hanosaurus (Wang et al., 2022) and numerous eosauropterygians (Rieppel, 1994). However, in contrast to Largocephalosaurus, the anterior margin of the humerus is not convex, but straight, making it more similar to the humeri of Placodus and Nothosaurus (Rieppel, 1994). The shape of the anterior margin of the humerus is unknown in Sinosaurosphargis. The posterior margin of the humerus is concave. The proximal and distal ends of the humerus are expanded, but a distinct humeral head and distal condyles are not present. Anteriorly, the distal end of the humerus bears a shallow ectepicondylar groove (notch), like in Largocephalosaurus qianensis (Li et al., 2014), but unlike in L. polycarpon (Cheng et al., 2012), which possesses an entepicondylar foramen. Anteroproximally, the exposed surface of the right humerus bears a short, straight groove, which likely represents a muscle insertion site.
Radius: A disarticulated right radius is completely preserved in HFUT YZSB-19-109 in close proximity to the right humerus and right pectoral girdle (Figure 3). The radius is proximodistally elongate and anteriorly curved, with a convex anterior margin and a concave posterior margin. The proximal and distal ends of the radius are straight and slightly expanded, with the proximal end being anteroposteriorly broader than the distal end. The radius is similar in general shape to that in other saurosphargids (Li et al., 2011; Li et al., 2014; Cheng et al., 2012), Majiashanosaurus (Jiang et al., 2014), Placodus (Jiang et al., 2008), and Helveticosaurus (Rieppel, 1989a), but differs from the straight and anteriorly and posteriorly concave radius of Eusaurosphargis (Scheyer et al., 2017). The radius/humerus proximodistal length ratio in HFUT YZSB-19-109 is ~0.55, in line with Eusaurosphargis (~0.51–0.57; Scheyer et al., 2017) and Placodus (~0.56; Jiang et al., 2008), but is significantly smaller than the ratio in Sinosaurosphargis (~0.66; Li et al., 2011), Largocephalosaurus polycarpon (~0.74; Cheng et al., 2012), L. qianensis (~0.67–0.72; Li et al., 2014), a referred specimen of Hanosaurus (~0.67; Wang et al., 2022), and Majiashanosaurus (~0.74; Jiang et al., 2014). This indicates that HFUT YZSB-19-109 had a proportionally shorter forearm compared with other saurosphargids and early eosauropterygians.
Pelvic girdle
Ilium: The left ilium is completely preserved in HFUT YZSB-19-109 and is exposed in lateral or medial view (Figure 5). The ilium consists of a distally expanded acetabular portion and a posteriorly projecting iliac blade, which is largely obscured by the overlying ischium. The ilium of HFUT YZSB-19-109 is very similar to the ilia of Largocephalosaurus (Li et al., 2014), Eusaurosphargis (Nosotti and Rieppel, 2003), Placodus (Rieppel, 1995), and Corosaurus (Storrs, 1991), which all possess a well-developed, posteriorly projecting iliac blade.
Pubis: The left pubis is completely preserved in HFUT YZSB-19-109 and exposed in ventral view, whereas only the distal portion of the right pubis is preserved (Figure 5). The pubis is approximately oval in outline, being proximodistally longer than anteroposteriorly broad and bears a posteroproximally positioned, open obturator foramen. The pubis of HFUT YZSB-19-109 resembles the pubis of Largocephalosaurus (Li et al., 2014) in outline and proportions. It is also similar to the pubis of the holotype and referred specimens of Hanosaurus (Rieppel, 1998a; Wang et al., 2022) and Pararcus diepenbroekii (Klein and Scheyer, 2013), which are however more circular in outline, being approximately as wide proximodistally as long anteroposteriorly. The pubis of HFUT YZSB-19-109 differs markedly from the anteriorly and posteriorly shallowly emarginated pubis of Eusaurosphargis (Scheyer et al., 2017) and Placodus (Drevermann, 1933), and the deeply emarginated pubis of eosauropterygians (e.g. Rieppel, 2000a).
Ischium: The left ischium of HFUT YZSB-19-109 is completely preserved, but is rotated 180° relative to its life position and is exposed in dorsal view, whereas only the distal portion of the right ischium is preserved (Figure 5). The ischium forms an anterodistally convex and posteroproximally concave plate, similar to that in Largocephalosaurus (Li et al., 2014), Pararcus (Klein and Scheyer, 2013) and Hanosaurus (Rieppel, 1998a; Wang et al., 2022), but differs markedly from the anteriorly emarginated ischium of Eusaurosphargis (Scheyer et al., 2017) and the anteriorly and posteriorly emarginated ischia of some placodonts and eosauropterygians (Rieppel, 2000a).
Hindlimb
Femur: The left hindlimb is completely preserved in HFUT YZSB-19-109. The femur is exposed in posterior view (Figure 5). The shaft of the femur is straight and both the proximal and distal ends are expanded. The internal trochanter is well-developed and located proximally, as in Largocephalosaurus (Li et al., 2014), Simosaurus, and Nothosaurus (Rieppel, 1994), but differs from the more distally located trochanter in the holotype of Hanosaurus (Rieppel, 1998a). Distally, the femur produces weakly-developed, but still distinct, condyles for the tibia and fibula, separated ventrally by a shallow popliteal area. The humerus/femur ratio in HFUT YZSB-19-109 is ~1.19, which is similar to the ratio in Largocephalosaurus (~1.20; Li et al., 2014), but is significantly greater than the ratio in Helveticosaurus (~1.11; Rieppel 1989), Placodus (~1.05; Jiang et al., 2008), and Eusaurosphargis (~0.89; Scheyer et al., 2017).
Tibia and fibula: The tibia is proximodistally slightly longer than the fibula and possesses proximally and distally expanded ends, with the proximal end slightly broader than the distal end (Figure 5). Posteroproximally, the tibia bears a proximodistally elongate, shallow facet for the fibula. The anterior and posterior margins of the tibia are gently concave. The fibula possesses an approximately straight posterior margin and a concave anterior margin (Figure 5). The proximal and distal ends of the fibula are slightly expanded, with the distal end being slightly broader than the proximal end. This is in contrast to Largocephalosaurus, in which the proximal end of the fibula is markedly broader than the distal end (Li et al., 2014).
Tarsals: Three tarsals are present in the left hindlimb of HFUT YZSB-19-109 – the largest one being the astragalus, the medium-sized representing the calcaneum, and the smallest element interpreted here as distal tarsal IV (Figure 5). The tarsals are sub-circular in outline, with the astragalus possessing minute notches anteriorly and posteriorly, and their exposed ventral surfaces are weakly concave. The morphology of the tarsals of HFUT YZSB-19-109 is similar to that in Largocephalosaurus, which, however, possesses four tarsals instead of three (astragalus, calcaneum and distal tarsals III and IV; Li et al., 2014).
Metatarsals: All five metatarsals are preserved in the left hindlimb of HFUT YZSB-19-109 (Figure 5). Metatarsal I is the proximodistally shortest metatarsal, being much broader proximally than distally. It is also the most robust of the metatarsals. Metatarsals II–V are slender, approximately hourglass-shaped in outline, with expaned proximal and distal ends. The proximodistal length of the metatarsals increases from metatarsal II–IV, with metatarsal IV being the longest of all metatarsals (similar to Largocephalosaurus polycarpon, but different from L. qianensis, in which metatarsal III is the longest; Li et al., 2014); the length of metatarsal V is comparable to that of metatarsal III. Metatarsal V is proportionally the most slender of the metatarsals (similar to the condition in L. qianensis, but unlike in L. polycarpon, in which metatarsal IV is the most slender; Li et al., 2014).
Phalanges: The pedal phalanges are completely preserved in the left pes of HFUT YZSB-19-109 (Figure 5). The proximal phalanges of digits 1 and 2 are sub-rectangular in outline, whereas those of digits 3–5 are hourglass-shaped and have expanded proximal and distal ends. Distally, the phalanges become proximodistally shorter and sub-rectangular in outline. The phalangeal formula is 2-3-4-5-5 (unguals 2 and 3 are slightly displaced).
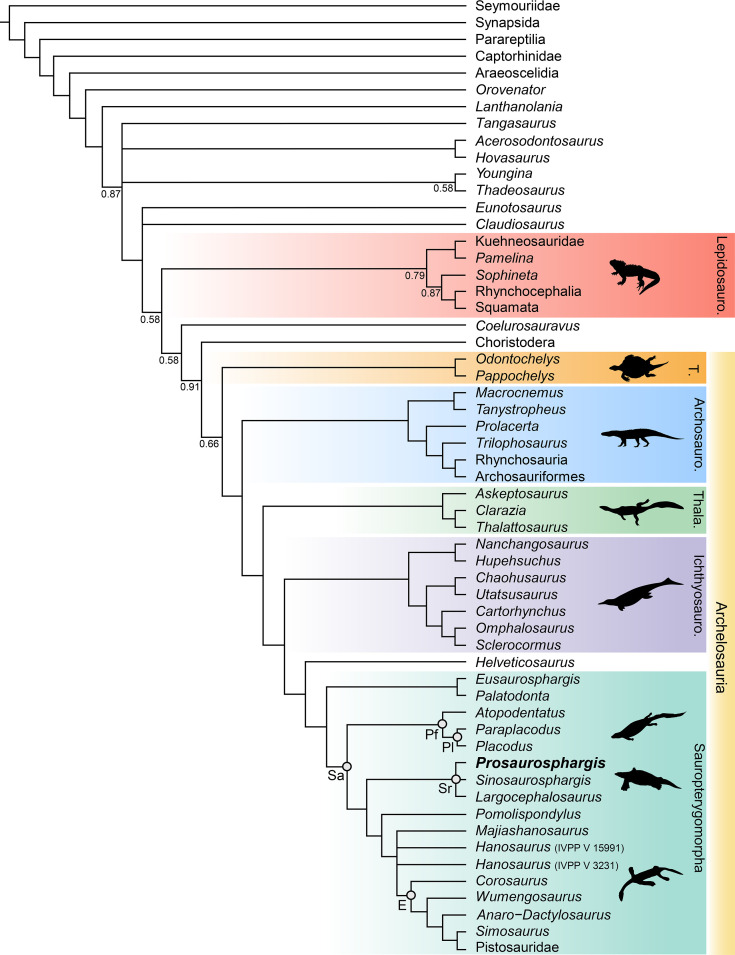
Phylogenetic results
The phylogenetic analysis recovered 48 most parsimonious trees (MPTs) of 1008 steps each (CI = 0.272, RI = 0.620) (Figure 8). Prosaurosphargis was recovered as a member of Saurosphargidae, forming a polytomy with Sinosaurosphargis and Largocephalosaurus. Saurosphargidae is supported by the following three unambiguous synapomorphies: dorsal ribs transversely broadened and in antero-posterior contact with each other, forming closed ‘rib-basket’ (char. 135.1); lateral gastralia expanded and flat (char. 141.1); distal end of ulna distinctly expanded (char. 167.1).
Figure 8. Phylogenetic relationships of Prosaurosphargis yingzishanensis within Diapsida.
The 50% majority rule consensus of 48 most parsimonious trees (MPTs) obtained from analysis of the updated dataset of Qiao et al., 2022. Numbers below nodes indicate proportion of MPTs in which the node is recovered if it is lower than 1. Abbreviations: Archosauro., Archosauromorpha; E, Eosauropterygia; Ichthyosauro., Ichthyosauromorpha; Lepidosauro., Lepidosauromorpha; Pf, Placodontiformes; Pl, Placodontia; Sa, Sauropterygia; Sr, Saurosphargidae; T., Testudines (total-group); Thala., Thalattosauria. All silhouettes are from Phylopic (http://phylopic.org).
© 2013, N Tamura
Ticinosuchus, Askeptosaurus, Utatsusaurus and Paraplacodus silhouettes were drawn by N. Tamura. Used with permission under license CC BY-NC 3.0 (https://creativecommons.org/licenses/by-nc/3.0/). Reproduction of this figure must abide by the terms of this license.
© 2013, G Monger
Keichousaurus silhouette was drawn by G Monger. Used with permission under license CC BY-NC 3.0 (https://creativecommons.org/licenses/by-nc/3.0/). Reproduction of this figure must abide by the terms of this license.
Saurosphargidae was recovered within Sauropterygia, as the sister-group to the lineage leading to Eosauropterygia. The saurosphargid-eosauropterygian clade is supported by six unambiguous synapomorphies: frontal, butterfly-shaped with antero- and postero-lateral processes absent (ch. 29.0); long postorbital posterior process contacting squamosal (ch. 32.0); mandibular articulations displaced to a level distinctly behind occipital condyle (ch. 87.1); neural canal evenly proportioned (ch. 122.0); three sacral ribs (ch. 130.1); total number of carpal ossifications more than three (ch. 194.0).
Pomolispondylus, recently proposed as a sister-taxon of Saurosphargidae (Cheng et al., 2022), was recovered as the most basal member of the grade leading to Eosauropterygia (Pomolispondylus + eosauropterygian lineage supported by a single unambiguous synapomorphy: transverse processes of neural arches of the dorsal region relatively short [ch. 124.0]). The type (Rieppel, 1998a) and referred (Wang et al., 2022) specimens of Hanosaurus were not unambiguously recovered in a monophyletic group – both specimens were recovered alongside Majiashanosaurus in a polytomy at the base of Eosauropterygia (clade comprising Hanosaurus, Majiashanosaurus, and Eosauropterygia supported by a single unambiguous synapomorphy: osteoderms absent [ch. 143.0]). Corosaurus was recovered as the earliest-diverging member of Eosauropterygia, whereas Wumengosaurus was recovered outside of the clade comprising pachypleurosaurs, nothosaurs and pistosaurs.
The herbivorous sauropterygian Atopodentatus was recovered as the sister-taxon of placodonts (represented in our dataset by Paraplacodus and Placodus) within Placodontiformes, supported by four unambiguous synapomorphies: contact of the prefrontal and postfrontal excluding frontal from dorsal orbital margin (char. 21.1); interpterygoid vacuity absent (char. 81.1); splenial entering mandibular symphysis (char. 88.0); and femur internal trochanter well developed (char. 203.0).
Palatodonta was recovered as the sister-taxon of Eusaurosphargis. This clade is supported by one unambiguous synapomorphy – a small premaxilla (char. 196.1). The clade comprising Palatodonta + Eusaurosphargis was recovered as the sister-group to Sauropterygia within Sauropterygomorpha tax. nov. (see above). Helveticosaurus was recovered as the sister-group of Sauropterygomorpha; the clade comprising Helveticosaurus + Sauropterygomorpha is supported by the following six unambiguous synapomorphies: preorbital and postorbital regions of skull of subequal length (ch. 1.0); transverse processes of neural arches of the dorsal region distinctly elongated (ch. 124.1); scapula with a constriction separating a ventral glenoidal portion from a posteriorly directed dorsal wing (ch. 154.2); distal tarsal I absent (ch. 184.1); total number of tarsal ossifications less than four (ch. 192.1); total number of carpal ossifications two (char. 194.2).
Within Diapsida, the clade comprising Helveticosaurus + Sauropterygomorpha was recovered as forming a clade with Ichthyosauromorpha, Thalattosauria and Archosauromorpha, a result similar to that recovered in some other recent broad-scale analyses of diapsid phylogenetic interrelationships (Chen et al., 2014a; Neenan et al., 2015; Martínez et al., 2021; Simões et al., 2022). The three major marine reptile clades (Sauropterygomorpha, Ichthyosauromorpha and Thalattosauria), Archosauromorpha, and Testudines were recovered within a monophyletic Archelosauria supported by four unambiguous synapomorphies – frontal with distinct posterolateral processes (ch. 26.1), frontal anterior margins oblique, forming an angle of at least 30 degrees with long axis of the skull (ch. 27.1), interclavicle anterior process or triangle conspicuously present (ch. 157.0), and upper temporal fossae present and distinctly smaller than the orbit (ch. 207.3).
Discussion
Marine reptile diversity of the Early Triassic Nanzhang-Yuan’an Fauna
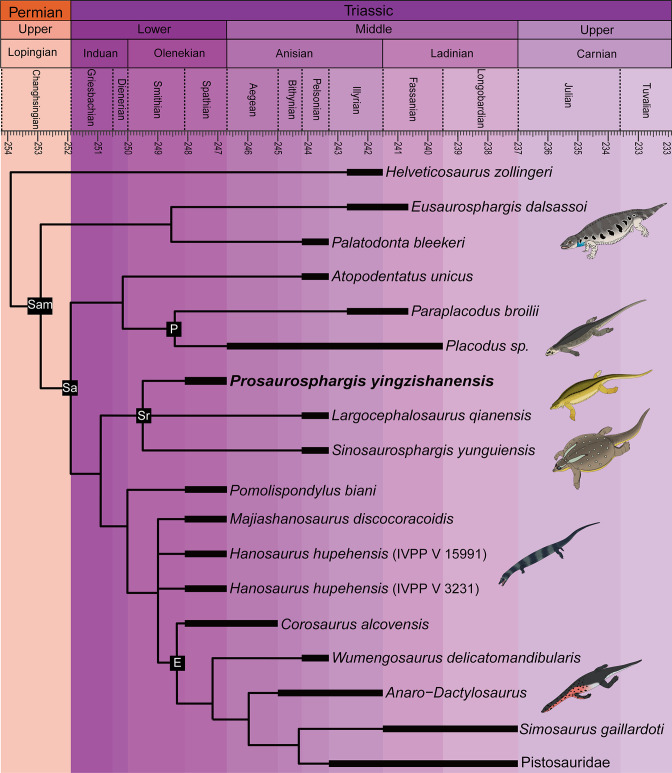
Prosaurosphargis represents the stratigraphically oldest occurrence of Saurosphargidae, extending their fossil record back by approximately 3 Ma from the Middle (Pelsonian) to the Early (Olenekian) Triassic (Figure 9). Saurosphargids are thus the fourth major marine reptile lineage known from the Early Triassic Nanzhang-Yuan’an fauna, which also includes as many as seven species of hupehsuchians (Chen et al., 2016a; Qiao et al., 2019), one species of ichthyosauriforms (Chen et al., 2013), and three taxa representing the sauropterygian lineage leading to Eosauropterygia – Hanosaurus, Lariosaurus sanxiaensis, and Pomolispondylus (Young, 1965; Rieppel, 1998a; Li and Liu, 2020; Cheng et al., 2022; Wang et al., 2022). Measuring approximately 1.5 m in total body length, Prosaurosphargis is one of the larger marine reptiles known from this ecosystem, smaller only than a large unidentified eosauropterygian (body length of 3–4 m; Chen et al., 2014b, Chen et al., 2016a) and an indeterminate hupehsuchian (body length of ~2.3 m; Qiao et al., 2019). The presence in the Nazhang-Yuan’an fauna of several marine reptiles representing a broad range of body sizes (~0.25–4.00 m; Chen et al., 2014b; Qiao et al., 2019) and displaying various ecomorphological adaptations supports the view of a rapid diversification of predators in the immediate aftermath of the PTME and high predation pressure in shallow marine ecosystems in the Early Triassic (Chen et al., 2014b; Chen et al., 2014c; Li and Liu, 2020).
Figure 9. Time-scaled phylogenetic tree of Sauropterygomorpha.
Abbreviations: E, Eosauropterygia; Pf, Placodontiformes; Pl, Placodontia; Sa, Sauropterygia; Sam, Sauropterygomorpha; Sr, Saurosphargidae.
Phylogeny of Sauropterygomorpha
A clade comprising Palatodonta + Eusaurosphargis and the lineage leading to Helveticosaurus are recovered as successive outgroups to Sauropterygia, with Palatodonta + Eusaurosphargis and Sauropterygia united within Sauropterygomorpha tax. nov. (see above) (Figure 9). The status of Eusaurosphargis + Palatodonta as the sister-group to Sauropterygia is corroborated by the presence in Eusaurosphargis of morphological features otherwise known exclusively in sauropterygians: a clavicle applied to the medial surface of the scapula and a pectoral fenestration (Scheyer et al., 2017). Furthermore, Eusaurosphargis and Sauropterygia also share a similar foot morphology with metatarsal I being proximodistally much shorter than metatarsal IV and metatarsal V being long and slender (see above). Helveticosaurus shares the presence of a skull with preorbital and postorbital regions subequal in length with Eusaurosphargis and Palatodonta, the presence of elongated dorsal transverse processes with Eusaurosphargis, placodonts and saurosphargids and the presence of a scapular constriction with Eusaurosphargis and sauropterygians. However, other anatomical features uniting it with Sauropterygomorpha include details of carpal and tarsal anatomy, which likely represent aquatic adaptations that might have evolved convergently in Helveticosaurus and aquatic representatives of Sauropterygomorpha (Chen et al., 2014a). Furthermore, Helveticosaurus lacks osteoderms, a feature present in early members of all major lineages within Sauropterygomorpha. Consequently, we interpret Helveticosaurus as a representative of a lineage closely related to Sauropterygomorpha but lying outside of it, that likely convergently evolved an aquatic lifestyle.
Our phylogenetic analysis does not recover Palatodonta from the Middle Triassic of the Netherlands as a sister-taxon of placodonts within Placodontiformes as was previously proposed (Neenan et al., 2013). Instead, Palatodonta is recovered as the sister-taxon of Eusaurosphargis. The Palatodonta + Eusaurosphargis clade recovered in our phylogenetic analysis is supported by one unambiguous synapomorphy – a small (anteroposteriorly short) premaxilla (ch. 196.0). In addition, both taxa also share anteriorly positioned external nares (ch. 197.0). Both of these character states are considered as typical for terrestrial taxa and contrast with the enlarged (anteroposteriorly elongate) premaxillae and posteriorly displaced external narial openings characteristic for marine reptiles (Chen et al., 2014a). A terrestrial lifetyle was previously proposed for Eusaurosphargis on the basis of manus and pes anatomy and bone microanatomy (Scheyer et al., 2017) and the femur of Eusaurosphargis is longer than its humerus (humerus:femur ratio ~0.89; Scheyer et al., 2017), which indicates hindlimb dominance characteristic of a terrestrial lifestyle (Motani and Vermeij, 2021). All this evidence strongly suggests that Palatodonta and Eusaurosphargis were terrestrial reptiles, likely representing the morphology of the last common terrestrial ancestor of Sauropterygia. Palatodonta is known from a single isolated skull (Neenan et al., 2013), whereas Eusaurosphargis is represented by two specimens with well preserved and largely complete postcrania, but only partially preserved skulls (Nosotti and Rieppel, 2003; Scheyer et al., 2017). Because postcranial remains referable to Eusaurosphargis were also reported from the type locality of Palatodonta, including a _Palatodonta_-like dentary preserved in close association with typical _Eusaurosphargis_-like vertebrae (Scheyer et al., 2019; Willemse et al., 2019), it is very likely that Palatodonta is a junior synonym of Eusaurosphargis, but the discovery of well-preserved skulls with associated postcranial elements of both of these taxa are needed to further test this hypothesis.
Atopodentatus and placodonts are recovered within Placodontiformes and this grouping is supported by four unambiguous synapomorphies (see above). Atopodentatus and the early-diverging placodonts Placodus and Paraplacodus possess a humerus which is longer than the femur, indicating a high level of adaptation to an aquatic lifestyle (Motani and Vermeij, 2021), but they also possess a massive femoral fourth trochanter and an ilium with a well-developed iliac blade (Jiang et al., 2008; Cheng et al., 2014). These features indicate that the hindlimbs in both Atopodentatus and placodonts were likely still important in locomotion at the bottom of the sea floor and/or on shore in a marginal marine environment and suggest a slightly lower degree of adaptation to an aquatic lifestyle in placodontiforms than in saurosphargids and eosauropterygians, in which the fourth trochanter is more reduced (Rieppel, 2000a; Li et al., 2014). The sister-group relationships of Atopodentatus and placodonts might also explain the absence of placodont fossils in Early Triassic fossil horizons worldwide. It is possible that the lineage leading to placodonts was represented in the Early Triassic by reptiles morphologically more similar to Atopodentatus than to placodonts and that the specialised placodont body plan did not evolve until the Middle Triassic. New discoveries of Early Triassic sauropterygians are likely to introduce new morphological data needed to test this hypothesis.
Pomolispondylus is not recovered as a sister-taxon of Saurosphargidae within Saurosphargiformes (contra Cheng et al., 2022), but as the most basal member of a grade of sauropterygians leading to Eosauropterygia. Such a phylogenetic position is supported by the presence in Pomolispondylus of dorsal transverse processes that are relatively short mediolaterally and broad anteroposteriorly, more similar in proportions to the dorsal transverse processes of Lariosaurus sanxiaensis (Li and Liu, 2020) and Hanosaurus (Wang et al., 2022) – two other representatives of the grade leading to Eosauropterygia – than to the mediolaterally broad and anteroposteriorly narrow dorsal neural spines of saurosphargids (Figure 3; Li et al., 2011; Li et al., 2014). Furthermore, Pomolispondylus possesses rows of rudimentary osteoderms on its body flanks, which are much more reduced than those present in Eusaurosphargis, early-diverging placodonts and saurosphargids (Klein and Scheyer, 2013; Li et al., 2014; Scheyer et al., 2017). Therefore, the osteoderms in Pomolispondylus likely represent a late stage of osteoderm reduction in the lineage leading to Eosauropterygia, rather than the first stages of osteoderm development in the saurosphargid lineage.
The type (Young, 1972; Rieppel, 1998a) and referred (Wang et al., 2022) specimens of Hanosaurus and Majiashanosaurus (Jiang et al., 2014) are also recovered in the paraphyletic grade leading to Eosauropterygia, but in a position more derived than Pomolispondylus. This result is in contrast to previous studies which recovered these taxa as either the outgroup to Saurosphargidae + Sauropterygia (Hanosaurus; Wang et al., 2022), pachypleurosaurs (Rieppel, 1998a; Neenan et al., 2015; Lin et al., 2021) or successive outgroups to a clade comprising Pachypleurosauria + Nothosauroidea to the exclusion of Pistosauroidea (Li and Liu, 2020). Suprisingly, the type specimen of Hanosaurus is not unambiguously recovered in a clade with the referred specimen. Taxonomic distinction of both specimens is supported by the fact that the anteriorly and posteriorly weakly emarginated coracoid of the type specimen of Hanosaurus (Figure 7G and H; Rieppel, 1998a; pers. obs. of IVPP V 3231) more closely resembles that of Corosaurus than the sub-oval coracoid present in the referred specimen of Hanosaurus (Figure 7I; Wang et al., 2022), which likely does not represent Hanosaurus, but is probably closely related or even referable to Lariosaurus sanxiaensis (Figure 7J; Chen et al., 2016b; Li and Liu, 2020). Corosaurus is recovered as the earliest-diverging eosauropterygian, a result similar to that obtained by Rieppel, 1994 and Li and Liu, 2020, but in contrast to some other phylogenetic analyses, which recovered it as a pistosauroid (Rieppel, 1998b; Wang et al., 2022) or the earliest-diverging eusauropterygian (Lin et al., 2021). Wumengosaurus is recovered as the sister-taxon of the clade comprising pachypleurosaurs, nothosaurs and pistosaurs, in contrast to a recent phylogenetic analysis which recovered it wtihin pachypleurosaurs (Xu et al., 2022), but similar to the phylogenetic results obtained by Wu et al., 2011 that recovered Wumengosaurus as the outgroup to a clade comprising pachypleurosaurs and nothosaurs.
The results of our phylogenetic analysis differ significantly from the results of a phylogenetic analysis recently published by Wang et al., 2022, in which Hanosaurus was recovered as the sister-group to a clade comprising Saurosphargidae + Sauropterygia within a monophyletic Sauropterygiformes. However, we believe that our phylogenetic analysis presents a more accurate topology of sauropterygians and their relatives for the following reasons. Wang et al., 2022 used 16 outgroup (non-sauropterygiform) taxa (15 marine reptiles and a single terrestrial reptile), representing five major reptile lineages, whereas our study included 40 outgroup (non-sauropterygomorph) taxa (19 terrestrial and 21 aquatic reptiles), representing 23 major reptile lineages. The inclusion of only a single terrestrial outgroup taxon – Youngina – in the analysis of Wang et al., 2022 is problematic because Youngina likely represents a taxon rather distantly related to the Mesozoic marine reptile clade which includes Sauropterygia (Figure 8; see also Simões et al., 2022). Distantly related taxa likely share fewer character states with derived taxa (homoplasy accumulation through time), so a phylogenetic analysis containing a single, distantly related outgroup taxon will likely fail to adequately capture important character transformation sequences, in contrast to a phylogenetic analysis in which outgroups are more comprehensively sampled (Wilberg, 2015). Furthermore, the phylogenetic analysis of Wang et al., 2022 used 181 morphological characters, in contrast to 221 characters included in our study. Greater character sampling has been demonstrated as an important factor increasing the accuracy of phylogenetic reconstructions (Wiens, 2006), which also favours the results obtained by our analysis.
The different topologies recovered by both studies are likely also partially a consequence of differences in character scoring. For example, Wang et al., 2022 scored the humerus of Hanosaurus as ‘rather straight’ (plesiomorphic state), similar to the humerus of Youngina and other non-sauropterygiform marine reptiles. However, in our opinion, the humerus morphology of Hanosaurus matches the typical ‘curved’ morphology (derived state) characteristic of saurosphargids, placodonts and the majority of eosauropterygians (Rieppel, 2000a). Wang et al., 2022 also scored Hanosaurus into an updated version of the phylogenetic matrix of Neenan et al., 2013. Hanosaurus was recovered as the earliest-diverging sauropterygiform in this analysis as well, but its humerus was also scored as plesiomorphic (‘rather straight’) in the character-taxon matrix. Interestingly, this updated analysis of Neenan et al., 2013 included a more comprehensive outgroup (non-sauropterygiform) sample (12 terrestrial and 5 aquatic taxa, representing 12 major reptile lineages) than the analysis of Wang et al., 2022 and recovered Eusaurosphargis and Helveticosaurus as successive sister-groups to Sauropterygia – a result similar to the one obtained in this study (Figure 9).
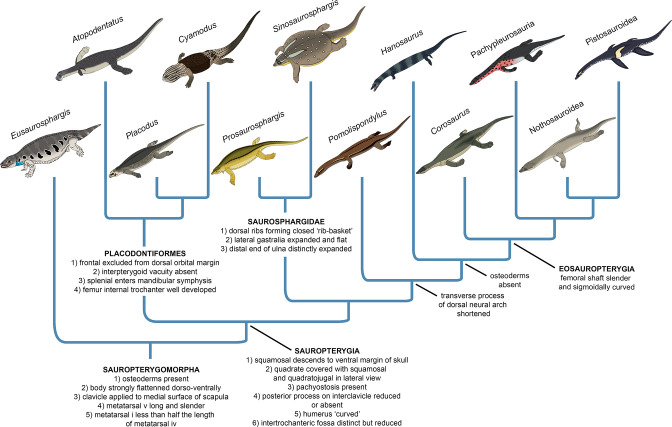
The early evolutionary assembly of the sauropterygian body plan
Our phylogenetic analysis suggests that Eusaurosphargis and Palatodonta likely represent the morphology of the last common terrestrial ancestor of sauropterygians, indicating it possessed well-developed dermal armour and the characteristic pectoral girdle and pes morphology that underwent further modifications in Sauropterygia. The topology recovered by our phylogenetic analysis demonstrates that the early evolution of sauropterygians first involved diversification within a shallow marine environment and exploration of various food resources, as evidenced by the disparate ecologies exhibited by Atopodentatus (herbivore), placodonts (durophages), saurosphargids, and early-diverging members of the eosauropterygian lineage (likely feeding on fish and invertebrates). Three key episodes can be identified in the evolution of the eosauropterygian body plan (Figure 10). The first, represented by Pomolispondylus, involved a reduction of osteoderms and shortening of the transverse processes of the dorsal neural spines, features well-developed in Eusaurosphargis, placodonts and saurosphargids. Majiashanosaurus and the referred specimen of Hanosaurus exemplify the second episode, in which osteoderms underwent complete reduction. The type specimen of Hanosaurus represents the earliest stage of the evolution of the characteristic eosauropterygian pectoral girdle morphology with an anteriorly and posteriorly emarginated coracoid that ultimately allowed eosauropterygians to become efficient, paraxial swimmers. The presence of the stratigraphically oldest saurosphargid (Prosaurosphargis), stratigraphically oldest representatives of the lineage leading to Eosauropterygia (Pomolispondylus, Hanosaurus, Majiashanosaurus), and the earliest-diverging placodontiform Atopodentatus in the Early–Middle Triassic of South China (Figure 9) indicates that sauropterygians likely originated and underwent rapid diversification in South China in the aftermath of the end-Smithian extinction, similar to ichthyosauromorphs (Motani et al., 2017; Moon and Stubbs, 2020), but well-constrained stratigraphic data for early sauropterygians are needed to further test this hypothesis.
Figure 10. Evolution of the sauropterygian body plan.
Simplified phylogeny of Sauropterygomorpha with key anatomical traits (synapomorphies reconstructed from the phylogenetic analysis) indicated for important nodes.
Our phylogenetic analysis indicates the important role of body armour in sauropterygian evolution (Figure 10). Dermal armour was likely an important preadaptation that allowed colonisation of the shallow marine realm by a _Eusaurosphargis_-like ancestor, enabling it to counteract buoyancy and walk on the bottom of the shallow sea in search of food (Houssaye, 2009). Elaboration of the dermal armour occurred in the shallow marine placodonts and saurosphargids, perhaps as a response to predation pressure (Liu et al., 2014; Chen et al., 2014b; Qiao et al., 2019). The reduction and complete loss of dermal armour then occurred in the lineage leading to Eosauropterygia, and was likely associated with the evolution of active predation, an efficient swimming style and increasing adaptation to a pelagic lifestyle. The evolution of the sauropterygian body plan demonstrates striking parallels with the evolution of the body plan of another important group of Mesozoic marine reptiles – the ichthyosaurs. Early-diverging representatives of Ichthyosauromorpha – hupehsuchians and omphalosaurids – also possesed a covering of osteoderms superficially similar to that present in Eusaurosphargis, early-diverging placodonts, and saurosphargids (Chen et al., 2014b; Jiang et al., 2016; Qiao et al., 2022). In the ichthyosauromorph lineage, the osteoderm covering was completely lost in Chaohusaurus, a basal ichthyosauriform that evolved an anguilliform mode of swimming and most likely a pelagic lifestyle (Motani et al., 1996; Nakajima et al., 2014; Qiao et al., 2022). This indicates that the evolutionary reduction of dermal armour in both sauropterygians and ichthyosauromorphs followed a similar pattern, probably in response to increasing adaptation to an aquatic lifestyle. These evolutionary parallels seem to demonstrate that the dermal body armour could have been a possible prerequsite (preadaptation) for the invasion of the shallow marine realm in different diapsid clades, which allowed for buoyancy reduction and exploration of the shallow marine environment in search of food. Fossils of terrestrial relatives of ichthyosauromorphs and thalattosaurs are needed to further test this evolutionary scenario.
Phylogenetic interrelationships within Diapsida
Our phylogenetic analysis is thus far one of only two phylogenetic analyses based on a morphology-only dataset of Diapsida that recovers a close relationship between Archosauromorpha and Testudines within a monophyletic Archelosauria (Figure 8) (see also Simões et al., 2022). A close phylogenetic relationship between Archosauromorpha and Testudines has been strongly supported by molecular data for the last twenty years, but was until recently not recovered by any phylogenetic analysis based entirely on morphological data, in which turtles were usually recovered as more closely related to lepidosauromorphs than archosauromorphs (reviewed in Lyson and Bever, 2020). Furthermore, our analysis recovers Eunotosaurus from the Permian of South Africa outside of Sauria (Figure 8), which is in agreement with the results obtained by Simões et al., 2022, but in contrast to all other recent phylogenetic analyses focussing on the phylogenetic interrelationships among Reptilia, which recovered Eunotosaurus as a stem turtle (Lyson et al., 2013; Bever et al., 2015; Li et al., 2018; Schoch and Sues, 2018). This indicates that the characteristic morphological features of the skull and postcranial skeleton shared between Eunotosaurus and early turtles, such as elongate vertebrae and broadened ribs, evolved convergently in both taxa. Archelosauria are supported in our analysis by four unambiguous synapomorphies associated with the morphology of the skull and shoulder girdle (see above). This demonstrates that innovations of the cranium linked with the evolution of sensory organs and the feeding apparatus, as well as changes in locomotion, perhaps underlie the evolutionary success of archelosaurian reptiles.
Conclusions
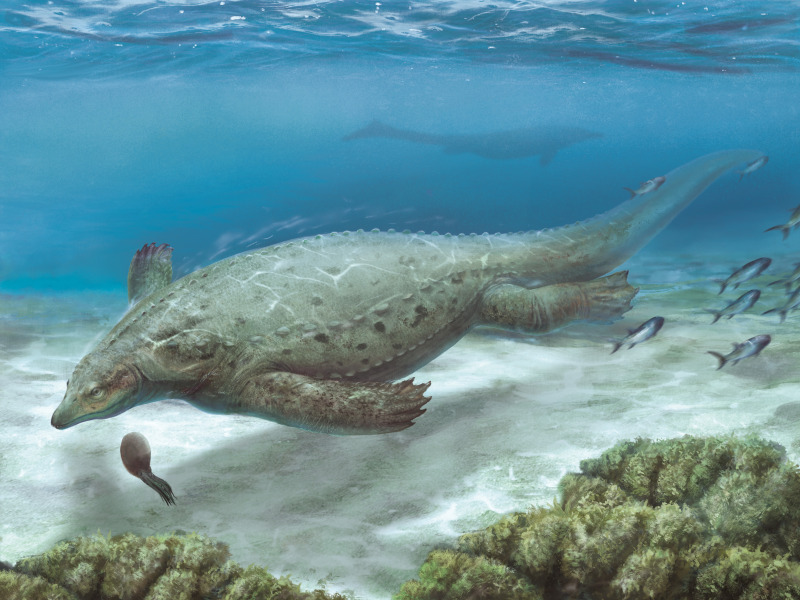
The new saurosphargid Prosaurosphargis yingzishanensis gen. et sp. nov. from the Early Triassic of South China (Figure 11) represents the earliest reported occurrence of Saurosphargidae, extending their temporal range back by 3 Ma. An updated phylogenetic analysis of Diapsida recovers saurosphargids as nested within Sauropterygia, forming a clade with Eosauropterygia to the exclusion of Placodontia. A clade comprising Eusaurosphargis and Palatodonta forms the sister-group to Sauropterygia within Sauropterygomorpha tax. nov. and their morphology likely represents the morphology of the last common terrestrial ancestor of Sauropterygia. The herbivorous sauropterygian Atopodentaus is recovered within Placodontiformes, whereas Pomolispondylus, Hanosaurus and Majishanosaurus form a grade at the base of Eosauropterygia, with the type and referred specimens of Hanosaurus likely representing distinct taxa. Our new phylogenetic hypothesis indicates sauropterygians originated and diversified in South China in the aftermath of the Permo-Triassic mass extinction event and suggests an important role of dermal armour in their early evolutionary history. Three major marine reptile clades – Sauropterygomorpha, Ichthyosauromorpha and Thalattosauria – are recovered within Archelosauria, together with Archosauromorpha and Testudines. Our study demonstrates the importance of including not only a broad sample of outgroup taxa in phylogenetic analyses, but also choosing their stratigraphically oldest and/or anatomically most plesiomorphic representatives as operational taxonomic units, in order to accurately reconstruct the phylogenetic relationships between major extant and extinct reptilian lineages.
Figure 11. Life reconstruction of Prosaurosphargis yingzishanensis depicted in the Early Triassic shallow marine environment of the Nanzhang-Yuan’an region, Hubei Province, South China.
Materials and methods
In order to investigate the phylogenetic position of Prosaurosphargis yingzishanensis, specimen HFUT YZSB-19-109 was scored into a modified version of a data matrix focusing on the phylogenetic interrelationships between the major groups of diapsid reptiles published by Qiao et al., 2022, which in itself is a modified version of the data matrix previously published by Jiang et al., 2016 and Chen et al., 2014a. The data matrix contains 57 OTUs (Operational Taxonomic Units) scored for a total of 221 characters – characters 1–220 are the original characters of Qiao et al., 2022 and character 221 was adapted from Li et al., 2014 (ch. 88) (Source data 1). In addition to Prosaurosphargis, 10 OTUs were added to the original dataset of Qiao et al., 2022: Eunotosaurus africanus (scored after Cox, 1969; Gow, 1997; Lyson et al., 2013; Lyson et al., 2016; Bever et al., 2015), Pappochelys rosinae (Schoch and Sues, 2015; Schoch and Sues, 2018); Hanosaurus hupehensis (type specimen) (Rieppel, 1998a and personal observation of specimen IVPP V 3231), Hanosaurus hupehensis (referred specimen) (Wang et al., 2022), Majiashanosaurus discocoracoidis (Jiang et al., 2014), Corosaurus alcovensis (Storrs, 1991; Rieppel, 1998b), Atopodentatus unicus (Cheng et al., 2014; Li et al., 2016), Palatodonta bleekeri (Neenan et al., 2013), Paraplacodus broilii (Peyer, 1935; Rieppel, 2000b), and Pomolispondylus biani (Cheng et al., 2022). This was done in order to include the majority of currently known Early Triassic sauropterygians in the data matrix and increase the sampling of early-diverging representatives of the main sauropterygian lineages, as well as their potential sister-groups. Furthermore, the holotype and referred specimen of Sclerocormus, included as separate OTUs in the dataset of Qiao et al., 2022, were merged into a single OTU in the current analysis.
Parsimony analysis of the data matrix was performed in TNT 1.5 (Goloboff and Catalano, 2016) using a Traditional Search algorithm (random seed = 1, replications of Wagner trees = 1000, number of trees saved per replication = 10), followed by an additional round of TBR branch-swapping. All characters were treated as equally weighted and unordered.
Acknowledgements
We thank PM Sander for early discussions, W Lin for sharing photographs of the Hanosaurus holotype, BY Xiang, XJ Liu and the HFUT Paleontology Lab members for assistance in the field, and the Willi Hennig Society for making the programme TNT publicly available. F Huang, L Li and T Sato skilfully prepared the fossil specimen. T Hollmann produced the skeletal reconstruction in Figure 2B and the sauropterygian cartoons in Figures 9 and 10, and Z Han provided the life reconstruction in Figure 11. The senior editor GH Perry, reviewing editor N Ibrahim, reviewer MJ Benton and another annoymous reviewer provided constructive comments that helped to improve the manuscipt.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Jun Liu, Email: junliu@hfut.edu.cn.
Nizar Ibrahim, University of Portsmouth, United Kingdom.
George H Perry, Pennsylvania State University, United States.
Funding Information
This paper was supported by the following grants:
- National Natural Science Foundation of China 42172026 to Jun Liu.
- National Natural Science Foundation of China 41772003 to Jun Liu.
- National Natural Science Foundation of China 42202006 to Andrzej S Wolniewicz.
- National Natural Science Foundation of China 41902104 to Yuefeng Shen.
- National Natural Science Foundation of China 41807333 to Yuanyuan Sun.
- Chengdu Center of China Geological Survey Liu Baojun Funding to Yuanyuan Sun.
- China Postdoc Council International Postdoctoral Exchange Fellowship Program to Andrzej S Wolniewicz.
Additional information
Competing interests
No competing interests declared.
Author contributions
Software, Formal analysis, Funding acquisition, Validation, Investigation, Visualization, Methodology, Writing – original draft, Writing – review and editing.
Formal analysis, Funding acquisition, Validation, Investigation, Methodology, Writing – original draft.
Formal analysis, Validation, Investigation, Visualization, Methodology.
Investigation.
Software, Formal analysis, Investigation, Methodology.
Software, Investigation, Visualization.
Investigation, Visualization.
Conceptualization, Resources, Data curation, Formal analysis, Supervision, Funding acquisition, Validation, Investigation, Methodology, Project administration, Writing – review and editing.
Additional files
MDAR checklist
Source data 1. Character-taxon matrix used in the phylogenetic analysis.
Data availability
Specimen HFUT YZSB-19-109 is housed in the collections of the Geological Museum, Hefei University of Technology, Hefei, China and available for examination upon request to JL. The phylogenetic data matrix used in this study is available in Source data 1.
References
- Bardet N. Extinction events among Mesozoic marine reptiles. Historical Biology. 1994;7:313–324. doi: 10.1080/10292389409380462. [DOI] [Google Scholar]
- Benson RBJ. In: Grzimek’s Animal Life Encyclopedia: Extinction. MacLeod N, Archibald JD, Levin P, editors. Gale Research Inc; 2013. Marine reptiles; pp. 267–279. [Google Scholar]
- Benson RBJ, Druckenmiller PS. Faunal turnover of marine tetrapods during the Jurassic-Cretaceous transition. Biological Reviews of the Cambridge Philosophical Society. 2014;89:1–23. doi: 10.1111/brv.12038. [DOI] [PubMed] [Google Scholar]
- Benton MJ. When Life Nearly Died: The Greatest Mass Extinction of All Time. Thames & Hudson; 2015. [Google Scholar]
- Bever GS, Lyson TR, Field DJ, Bhullar BAS. Evolutionary origin of the turtle skull. Nature. 2015;525:239–242. doi: 10.1038/nature14900. [DOI] [PubMed] [Google Scholar]
- Bureau of Geology and Mineral Resources of Hubei Province . Regional Geology of Hubei Province. Geological Publishing House; 1990. [Google Scholar]
- Chen XH, Sander PM, Cheng L, Wang XF. A new Triassic primitive ichthyosaur from Yuanan, South China. Acta Geologica Sinica. 2013;87:672–677. doi: 10.1111/1755-6724.12078. [DOI] [Google Scholar]
- Chen XH, Motani R, Cheng L, Jiang DY, Rieppel O. The enigmatic marine reptile Nanchangosaurus from the Lower Triassic of Hubei, China and the phylogenetic affinities of Hupehsuchia. PLOS ONE. 2014a;9:e102361. doi: 10.1371/journal.pone.0102361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Motani R, Cheng L, Jiang D, Rieppel O. A carapace-like bony “body tube” in an early triassic marine reptile and the onset of marine tetrapod predation. PLOS ONE. 2014b;9:e94396. doi: 10.1371/journal.pone.0094396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Motani R, Cheng L, Jiang D, Rieppel O. A small short-necked hupehsuchian from the lower Triassic of Hubei Province, China. PLOS ONE. 2014c;9:e115244. doi: 10.1371/journal.pone.0115244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Chen XH, Cheng L, Yan CB. Nangzhang-Yuan’an Fauna, Hubei Province and its significance for biotic recovery. Acta Geologica Sinica. 2016a;90:409–420. [Google Scholar]
- Chen XH, Cheng L, Wang C, Zhang B. Triassic Marine Reptile Faunas from Middle and Upper Yangtze Areas and Their Coevolution with Environment. Geological Publishing House; 2016b. [Google Scholar]
- Chen Y, Jiang H, Ogg JG, Zhang Y, Gong Y, Yan C. Early-Middle Triassic boundary interval: Integrated chemo-bio-magneto-stratigraphy of potential GSSPs for the base of the Anisian Stage in South China. Earth and Planetary Science Letters. 2020;530:115863. doi: 10.1016/j.epsl.2019.115863. [DOI] [Google Scholar]
- Chen Y, Shen Y, Liu J. Carbonate laminites from the Nanzhang-Yuanan fauna in the Lower Triassic Jialingjiang Formation, South China: origins and significances. The 21st International Sedimentological Congress.2022. [Google Scholar]
- Cheng L, Chen X, Zeng X, Cai Y. A new eosauropterygian (Diapsida: Sauropterygia) from the Middle Triassic of Luoping, Yunnan Province. Journal of Earth Science. 2012;23:33–40. doi: 10.1007/s12583-012-0231-z. [DOI] [Google Scholar]
- Cheng L, Chen X, Shang Q, Wu X. A new marine reptile from the Triassic of China, with A highly specialized feeding adaptation. Die Naturwissenschaften. 2014;101:251–259. doi: 10.1007/s00114-014-1148-4. [DOI] [PubMed] [Google Scholar]
- Cheng L, Yan C, Chen X, Zeng X, Motani R. Characteristics and significance of Nanzhang/Yuanan Fauna, Hubei Province. Geology in China. 2015;42:676–684. [Google Scholar]
- Cheng L, Motani R, Jiang DY, Yan CB, Tintori A, Rieppel O. Early Triassic marine reptile representing the oldest record of unusually small eyes in reptiles indicating non-visual prey detection. Scientific Reports. 2019;9:152. doi: 10.1038/s41598-018-37754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, C. Moon B, Yan C, Motani R, Jiang D, An Z, Fang Z. The oldest record of Saurosphargiformes (Diapsida) from South China could fill an ecological gap in the Early Triassic biotic recovery. PeerJ. 2022;10:e13569. doi: 10.7717/peerj.13569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CB. The problematic permian reptile eunotosaurus. Bulletin of the British Museum (Natural History) Geology. 1969;18:165–196. [Google Scholar]
- Crawford NG, Parham JF, Sellas AB, Faircloth BC, Glenn TC, Papenfuss TJ, Henderson JB, Hansen MH, Simison WB. A phylogenomic analysis of turtles. Molecular Phylogenetics and Evolution. 2015;83:250–257. doi: 10.1016/j.ympev.2014.10.021. [DOI] [PubMed] [Google Scholar]
- Crofts SB, Neenan JM, Scheyer TM, Summers AP. Tooth occlusal morphology in the durophagous marine reptiles, Placodontia (Reptilia: Sauropterygia) Paleobiology. 2017;43:114–128. doi: 10.1017/pab.2016.27. [DOI] [Google Scholar]
- deBraga M, Rieppel O. Reptile phylogeny and the interrelationships of turtles. Zoological Journal of the Linnean Society. 1997;120:281–354. doi: 10.1111/j.1096-3642.1997.tb01280.x. [DOI] [Google Scholar]
- Drevermann FR. Die Placodontier. 3. Das Skelett von Placodus gigas Agassiz im Senckenberg-Museum. Abhandlungender Senckenbergischen Naturforschenden Gesellschaft. 1933;38:319–364. [Google Scholar]
- Goloboff PA, Catalano SA. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics. 2016;32:221–238. doi: 10.1111/cla.12160. [DOI] [PubMed] [Google Scholar]
- Gow CE. A reassessment of Eunotosaurus africanus Seeley (Amniota: Parareptilia) Palaeontologia Africana. 1997;34:33–42. [Google Scholar]
- Hirasawa T, Nagashima H, Kuratani S. The endoskeletal origin of the turtle carapace. Nature Communications. 2013;4:2107. doi: 10.1038/ncomms3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houssaye A. “Pachyostosis” in aquatic amniotes: a review. Integrative Zoology. 2009;4:325–340. doi: 10.1111/j.1749-4877.2009.00146.x. [DOI] [PubMed] [Google Scholar]
- Huene F. Henodus chelyops, ein neuer Placodontier. Palaeontographica A. 1936;84:99–148. [Google Scholar]
- Ji C, Jiang DY, Rieppel O, Motani R, Tintori A, Sun ZY. A new specimen of Nothosaurus youngi from the Middle Triassic of Guizhou, China. Journal of Vertebrate Paleontology. 2014;34:465–470. doi: 10.1080/02724634.2013.808204. [DOI] [Google Scholar]
- Jiang D, Motani R, Hao W, Rieppel O, Sun Y, Schmitz L, Sun Z. First record of Placodontoidea (Reptilia, Sauropterygia, Placodontia) from the Eastern Tethys. Journal of Vertebrate Paleontology. 2008;28:904–908. doi: 10.1671/0272-4634(2008)28[904:FROPRS]2.0.CO;2. [DOI] [Google Scholar]
- Jiang D, Motani R, Tintori A, Rieppel O, Chen G, Huang J, Zhang R, Sun Z, Ji C. The early Triassic eosauropterygian Majiashanosaurus discocoracoidis, gen. Journal of Vertebrate Paleontology. 2014;34:1044–1052. doi: 10.1080/02724634.2014.846264. [DOI] [Google Scholar]
- Jiang D, Motani R, Huang J, Tintori A, Hu Y, Rieppel O, Fraser NC, Ji C, Kelley NP, Fu W, Zhang R. A large aberrant stem ichthyosauriform indicating early rise and demise of ichthyosauromorphs in the wake of the end-Permian extinction. Scientific Reports. 2016;6:26232. doi: 10.1038/srep26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley NP, Motani R, Jiang D, Rieppel O, Schmitz L. Selective extinction of Triassic marine reptiles during long-term sea-level changes illuminated by seawater strontium isotopes. Palaeogeography, Palaeoclimatology, Palaeoecology. 2014;400:9–16. doi: 10.1016/j.palaeo.2012.07.026. [DOI] [Google Scholar]
- Kelley NP, Pyenson ND. Vertebrate evolution. Evolutionary innovation and ecology in marine tetrapods from the Triassic to the Anthropocene. Science. 2015;348:aaa3716. doi: 10.1126/science.aaa3716. [DOI] [PubMed] [Google Scholar]
- Klein N. Postcranial morphology and growth of the pachypleurosaur Anarosaurus heterodontus (Sauropterygia) from the Lower Muschelkalk of Winterswijk, The Netherlands. Paläontologische Zeitschrift. 2012;86:389–408. doi: 10.1007/s12542-012-0137-1. [DOI] [Google Scholar]
- Klein N, Scheyer TM. A new placodont sauropterygian from the Middle Triassic of the Netherlands. Acta Palaeontologica Polonica. 2013;59:887–902. doi: 10.4202/app.2012.0147. [DOI] [Google Scholar]
- Klein N, Eggmaier S, Hagdorn H. The redescription of the holotype of Nothosaurus mirabilis (Diapsida, Eosauropterygia)-a historical skeleton from the Muschelkalk (Middle Triassic, Anisian) near Bayreuth (southern Germany) PeerJ. 2022;10:e13818. doi: 10.7717/peerj.13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenti JN. Josephi Nicolai Laurenti… Specimen Medicum: Exhibens Synopsin Reptilium Emendatam Cum Experimentis circa Venena et Antidota Reptilium Austriacorum. Trattnern; 1768. [DOI] [Google Scholar]
- Li J, Liu J, Li C, Huang Z. The horizon and age of the marine reptiles from Hubei Province, China. Vertebrata Palasiatica. 2002;40:241–244. [Google Scholar]
- Li C, Rieppel O, Wu X, Zhao L, Wang L. A new Triassic marine reptile from southwestern China. Journal of Vertebrate Paleontology. 2011;31:303–312. doi: 10.1080/02724634.2011.550368. [DOI] [Google Scholar]
- Li C, Jiang D, Cheng L, Wu X, Rieppel O. A new species of Largocephalosaurus (Diapsida: Saurosphargidae), with implications for the morphological diversity and phylogeny of the group. Geological Magazine. 2014;151:100–120. doi: 10.1017/S001675681300023X. [DOI] [Google Scholar]
- Li C, Rieppel O, Cheng L, Fraser NC. The earliest herbivorous marine reptile and its remarkable jaw apparatus. Science Advances. 2016;2:e1501659. doi: 10.1126/sciadv.1501659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Fraser NC, Rieppel O, Wu X. A Triassic stem turtle with an edentulous beak. Nature. 2018;560:476–479. doi: 10.1038/s41586-018-0419-1. [DOI] [PubMed] [Google Scholar]
- Li Q, Liu J. An Early Triassic sauropterygian and associated fauna from South China provide insights into Triassic ecosystem health. Communications Biology. 2020;3:63. doi: 10.1038/s42003-020-0778-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhao B, Zou Y, Chen G. Research status of Hupehsuchia and preliminary understanding of their environmental evolution. Resources Environment & Engineering. 2020;34:485–493. [Google Scholar]
- Lin W, Jiang D, Rieppel O, Motani R, Tintori A, Sun Z, Zhou M. Panzhousaurus rotundirostris Jiang et al., 2019 (Diapsida: Sauropterygia) and the recovery of the monophyly of Pachypleurosauridae. Journal of Vertebrate Paleontology. 2021;41:e1901730. doi: 10.1080/02724634.2021.1901730. [DOI] [Google Scholar]
- Liu J, Hu S, Rieppel O, Jiang D, Benton MJ, Kelley NP, Aitchison JC, Zhou C, Wen W, Huang J, Xie T, Lv T. A gigantic nothosaur (Reptilia: Sauropterygia) from the Middle Triassic of SW China and its implication for the Triassic biotic recovery. Scientific Reports. 2014;4:7142. doi: 10.1038/srep07142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyson TR, Bever GS, Scheyer TM, Hsiang AY, Gauthier JA. Evolutionary origin of the turtle shell. Current Biology. 2013;23:1113–1119. doi: 10.1016/j.cub.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Lyson TR, Rubidge BS, Scheyer TM, de Queiroz K, Schachner ER, Smith RMH, Botha-Brink J, Bever GS. Fossorial origin of the turtle shell. Current Biology. 2016;26:1887–1894. doi: 10.1016/j.cub.2016.05.020. [DOI] [PubMed] [Google Scholar]
- Lyson TR, Bever GS. Origin and evolution of the turtle body plan. Annual Review of Ecology, Evolution, and Systematics. 2020;51:143–166. doi: 10.1146/annurev-ecolsys-110218-024746. [DOI] [Google Scholar]
- Martínez RN, Simões TR, Sobral G, Apesteguía S. A Triassic stem lepidosaur illuminates the origin of lizard-like reptiles. Nature. 2021;597:235–238. doi: 10.1038/s41586-021-03834-3. [DOI] [PubMed] [Google Scholar]
- Meyer H. Mittheilung, an Professor Bronn gerichtet. Neues Jahrbuch Für Mineralogie, Geognosie, Geologie Und Petrefaktenkunde. 1839;1839:559–560. [Google Scholar]
- Moon BC, Stubbs TL. Early high rates and disparity in the evolution of ichthyosaurs. Communications Biology. 2020;3:68. doi: 10.1038/s42003-020-0779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motani R, Yu H, McGowan C. Eel-like swimming in the earliest ichthyosaurs. Nature. 1996;382:347–348. doi: 10.1038/382347a0. [DOI] [Google Scholar]
- Motani R. The evolution of marine reptiles. Evolution. 2009;2:224–235. doi: 10.1007/s12052-009-0139-y. [DOI] [Google Scholar]
- Motani R, Jiang D, Chen G, Tintori A, Rieppel O, Ji C, Huang J. A basal ichthyosauriform with a short snout from the Lower Triassic of China. Nature. 2015;527:485–488. doi: 10.1038/nature15533. [DOI] [PubMed] [Google Scholar]
- Motani R, Jiang DY, Tintori A, Ji C, Huang JD. Pre- versus post-mass extinction divergence of Mesozoic marine reptiles dictated by time-scale dependence of evolutionary rates. Proceedings. Biological Sciences. 2017;284:20170241. doi: 10.1098/rspb.2017.0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motani R, Vermeij GJ. Ecophysiological steps of marine adaptation in extant and extinct non-avian tetrapods. Biological Reviews of the Cambridge Philosophical Society. 2021;96:1769–1798. doi: 10.1111/brv.12724. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Houssaye A, Endo H. Osteohistology of the Early Triassic ichthyopterygian reptile Utatsusaurus hataii: Implications for early ichthyosaur biology. Acta Palaeontologica Polonica. 2014;59:343–352. [Google Scholar]
- Neenan JM, Klein N, Scheyer TM. European origin of placodont marine reptiles and the evolution of crushing dentition in Placodontia. Nature Communications. 2013;4:1621. doi: 10.1038/ncomms2633. [DOI] [PubMed] [Google Scholar]
- Neenan JM, Li C, Rieppel O, Scheyer TM. The cranial anatomy of Chinese placodonts and the phylogeny of Placodontia (Diapsida: Sauropterygia) Zoological Journal of the Linnean Society. 2015;175:415–428. doi: 10.1111/zoj.12277. [DOI] [Google Scholar]
- Nosotti S, Rieppel O. Eusaurosphargis dalsassoi N. gen N. sp., a new, unusual diapsid reptile from the Middle Triassic of Besano (Lombardy, N Italy) Memorie Della Società Italiana Di Scienze Naturali e Del Museo Civico Di Storia Naturale Di Milano. 2003;31:3–33. [Google Scholar]
- Osborn HF. The reptilian subclasses Diapsida and Synapsida and the early history of the Diaptosauria. Memoirs of the American Museum of Natural History. 1903;1:449–507. [Google Scholar]
- Owen R. Palaeontology; or, a Systematic Summary of Extinct Animals and Their Geologic Remains. Adam and Charles Black, Edinburgh; 1860. [DOI] [Google Scholar]
- Peyer B. Die Triasfauna der Tessiner Kalkalpen VIII Weitere Placodontierfunde. Abhandlungen Der Schweizerischen Palaontologischen Gesellschaft. 1935;55:1–26. [Google Scholar]
- Qiao Y, Iijima M, Liu J. The largest hupehsuchian (Reptilia, Ichthyosauromorpha) from the Lower Triassic of South China indicates early establishment of high predation pressure after the Permo-Triassic mass extinction. Journal of Vertebrate Paleontology. 2019;39:e1719122. doi: 10.1080/02724634.2019.1719122. [DOI] [Google Scholar]
- Qiao Y, Liu J, Wolniewicz AS, Iijima M, Shen Y, Wintrich T, Li Q, Sander PM. A globally distributed durophagous marine reptile clade supports the rapid recovery of pelagic ecosystems after the Permo-Triassic mass extinction. Communications Biology. 2022;5:1242. doi: 10.1038/s42003-022-04162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieppel O. Helveticosaurus zollingeri Peyer (Reptilia, Diapsida) skeletal paedomorphosis, functional anatomy and systematic affinities. Palaeontographica. Abteilung A, Paläozoologie, Stratigraphie. 1989a;208:123–152. [Google Scholar]
- Rieppel O. A new pachypleurosaur (Reptilia: Sauropterygia) from the Middle Triassic of Monte San Giorgio, Switzerland. Philosophical Transactions of the Royal Society of London. B, Biological Sciences. 1989b;323:1–73. doi: 10.1098/rstb.1989.0001. [DOI] [PubMed] [Google Scholar]
- Rieppel O. Osteology of Simosaurus gaillardoti, and the phylogenetic interrelationships of stem-group Sauropterygia. Fieldiana. 1994;28:1–85. [Google Scholar]
- Rieppel O. The Genus Placodus: Systematics, Morphology, Paleobiogeography, and Paleobiology. Fieldiana (Geology; 1995. [DOI] [Google Scholar]
- Rieppel O. The systematic status of Hanosaurus hupehensis (Reptilia, Sauropterygia) from the Triassic of China. Journal of Vertebrate Paleontology. 1998a;18:545–557. doi: 10.1080/02724634.1998.10011082. [DOI] [Google Scholar]
- Rieppel O. Corosaurus alcovensis Case and the phylogenetic interrelationships of Triassic stem-group Sauropterygia. Zoological Journal of the Linnean Society. 1998b;124:1–41. doi: 10.1111/j.1096-3642.1998.tb00568.x. [DOI] [Google Scholar]
- Rieppel O. In: Encyclopedia of Paleoherpetology. Wellnhofer P, editor. Verlag Dr. Friedrich Pfeil; 2000a. Sauropterygia I; pp. 1–134. [Google Scholar]
- Rieppel O. Paraplacodus and the phylogeny of the Placodontia (Reptilia: Sauropterygia) Zoological Journal of the Linnean Society. 2000b;130:635–659. doi: 10.1111/j.1096-3642.2000.tb02204.x. [DOI] [Google Scholar]
- Rieppel O. Feeding mechanics in Triassic stem-group sauropterygians: the anatomy of a successful invasion of Mesozoic seas. Zoological Journal of the Linnean Society. 2002;135:33–63. doi: 10.1046/j.1096-3642.2002.00019.x. [DOI] [Google Scholar]
- Sander PM, Rieppel OC, Bucher H. A new pistosaurid (Reptilia: Sauropterygia) from the Middle Triassic of Nevada and its implications for the origin of the plesiosaurs. Journal of Vertebrate Paleontology. 1997;17:526–533. doi: 10.1080/02724634.1997.10010999. [DOI] [Google Scholar]
- Sander PM, Griebeler EM, Klein N, Juarbe JV, Wintrich T, Revell LJ, Schmitz L. Early giant reveals faster evolution of large body size in ichthyosaurs than in cetaceans. Science. 2021;374:eabf5787. doi: 10.1126/science.abf5787. [DOI] [PubMed] [Google Scholar]
- Scheyer TM. Skeletal histology of the dermal armor of Placodontia: the occurrence of “postcranial fibro-cartilaginous bone” and its developmental implications. Journal of Anatomy. 2007;211:737–753. doi: 10.1111/j.1469-7580.2007.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheyer TM, Romano C, Jenks J, Bucher H. Early Triassic marine biotic recovery: the predators’ perspective. PLOS ONE. 2014;9:e88987. doi: 10.1371/journal.pone.0088987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheyer TM, Neenan JM, Bodogan T, Furrer H, Obrist C, Plamondon M. A new, exceptionally preserved juvenile specimen of Eusaurosphargis dalsassoi (Diapsida) and implications for Mesozoic marine diapsid phylogeny. Scientific Reports. 2017;7:4406. doi: 10.1038/s41598-017-04514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheyer TM, Klein N, Sichelschmidt O, Neenan JM, Albers P. With plates and spikes-the heavily armoured Eusaurosphargis aff dalsassoi. Staringia. 2019;16:216–222. [Google Scholar]
- Scheyer TM, Oberli U, Klein N, Furrer H. A large osteoderm-bearing rib from the Upper Triassic Kössen Formation (Norian/Rhaetian) of eastern Switzerland. Swiss Journal of Palaeontology. 2022;141:1. doi: 10.1186/s13358-022-00244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch RR, Sues HD. A Middle Triassic stem-turtle and the evolution of the turtle body plan. Nature. 2015;523:584–587. doi: 10.1038/nature14472. [DOI] [PubMed] [Google Scholar]
- Schoch RR, Sues HD. Osteology of the Middle Triassic stem-turtle Pappochelys rosinae and the early evolution of the turtle skeleton. Journal of Systematic Palaeontology. 2018;16:927–965. doi: 10.1080/14772019.2017.1354936. [DOI] [Google Scholar]
- Shang X, Wu X, Li C. A new eosauropterygian from Middle Triassic of eastern Yunnan Province, southwestern China. Vertebrata PalAsiatica. 2011;49:155–171. [Google Scholar]
- Simões TR, Caldwell MW, Tałanda M, Bernardi M, Palci A, Vernygora O, Bernardini F, Mancini L, Nydam RL. The origin of squamates revealed by a Middle Triassic lizard from the Italian Alps. Nature. 2018;557:706–709. doi: 10.1038/s41586-018-0093-3. [DOI] [PubMed] [Google Scholar]
- Simões TR, Kammerer CF, Caldwell MW, Pierce SE. Successive climate crises in the deep past drove the early evolution and radiation of reptiles. Science Advances. 2022;8:eabq1898. doi: 10.1126/sciadv.abq1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrs GW. Anatomy and relationships of Corosaurus alcovensis (Diapsida: Sauropterygia) and the Triassic Alcova limestone of Wyoming. Bulletin of the Peabody Museum of Natural History. 1991;44:1–151. [Google Scholar]
- Stubbs TL, Benton MJ. Ecomorphological diversifications of Mesozoic marine reptiles: the roles of ecological opportunity and extinction. Paleobiology. 2016;42:547–573. doi: 10.1017/pab.2016.15. [DOI] [Google Scholar]
- Vermeij GJ, Motani R. Land to sea transitions in vertebrates: the dynamics of colonization. Paleobiology. 2018;44:237–250. doi: 10.1017/pab.2017.37. [DOI] [Google Scholar]
- Wang J, Cheng L, Zeng X. Sedimentary characteristics of the second member of Jialingjiang formation in the Lower Triassic of the Middle Yangtze Area and its reflection on the burial environment of marine reptiles. Abstract Volume of the 26th Annual Academic Meeting of the Palaeontological Society of China.2011. [Google Scholar]
- Wang W, Li C, Wu X. An adult specimen of Sinocyamodus xinpuensis (Sauropterygia: Placodontia) from Guanling, Guizhou, China. Zoological Journal of the Linnean Society. 2019;185:910–924. doi: 10.1093/zoolinnean/zly080. [DOI] [Google Scholar]
- Wang W, Ma F, Li C, Ruta M. First subadult specimen of Psephochelys polyosteoderma (Sauropterygia, Placodontia) implies turtle‐like fusion pattern of the carapace. Papers in Palaeontology. 2020;6:251–264. doi: 10.1002/spp2.1293. [DOI] [Google Scholar]
- Wang W, Shang Q, Cheng L, Wu X, Li C. Ancestral body plan and adaptive radiation of sauropterygian marine reptiles. iScience. 2022;25:105635. doi: 10.1016/j.isci.2022.105635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens JJ. Missing data and the design of phylogenetic analyses. Journal of Biomedical Informatics. 2006;39:34–42. doi: 10.1016/j.jbi.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Wilberg EW. What’s in an Outgroup? The Impact of Outgroup Choice on the Phylogenetic Position of Thalattosuchia (Crocodylomorpha) and the Origin of Crocodyliformes. Systematic Biology. 2015;64:621–637. doi: 10.1093/sysbio/syv020. [DOI] [PubMed] [Google Scholar]
- Willemse DM, Willemse NW, Voeten DFAE. An aff. Eusaurosphargis vertebra associated with an isolated dentary. Staringia. 2019;16:273–277. [Google Scholar]
- Wu X, Cheng Y, Li C, Zhao L, Sato T. New information on Wumengosaurus delicatomandibularis Jiang et al., 2008 (Diapsida: Sauropterygia), with a revision of the osteology and phylogeny of the taxon. Journal of Vertebrate Paleontology. 2011;31:70–83. [Google Scholar]
- Xu G, Ren Y, Zhao L, Liao J, Feng D. A long-tailed marine reptile from China provides new insights into the Middle Triassic pachypleurosaur radiation. Scientific Reports. 2022;12:7396. doi: 10.1038/s41598-022-11309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Cheng L, Tintori A, Li Z, Yang B. Stratigraphic characterization and paleoenvironmental analysis of Early Triassic Nanzhang-Yuan’an Fauna, western Hubei Province. Abstract Volume of Joint Meetings on the 12th National Congress and 29th Annual Academic Meeting of the Palaeontological Society of China; 2018. pp. 237–238. [Google Scholar]
- Yan C, Li J, Cheng L, Zhao B, Zou Y, Niu D, Chen G, Fang Z. Strata characteristics of the Early Triassic Nanzhang-Yuan’an Fauna in Western Hubei Province. Earth Science. 2021;46:122–135. [Google Scholar]
- Young CC. On the new nothosaurs from Hupeh and Kweichou, China. Vertebrata PalAsiatica. 1965;9:337–356. [Google Scholar]
- Young CC. A marine lizard from Nanchang, Hupeh Province. Memoirs of the Institute of Vertebrate Paleontology and Paleoanthropology. Academia Sinica. 1972;9:17–28. [Google Scholar]
- Zhao X, Tong J, Yao H, Zhang K, Zq C. Anachronistic facies in the Lower Triassic of South China and their implications to the ecosystems during the recovery time. Science in China Series D. 2008;51:1646–1657. doi: 10.1007/s11430-008-0128-y. [DOI] [Google Scholar]
- Zhao B, Zhou Y, Chen G, Li J, Wu K, Wan S, Xu X. New research progress on a specimen of Chaohusaurus zhangjiawanensis (diapsida: ichthyosauromorpha). Acta Geologica Sinica; 2022. pp. 769–782. [Google Scholar]
This significant new study is built around a remarkable fossil of a new genus and species of armoured marine reptile from the Early Triassic of China. More importantly, this paper also adds to our understanding of the diversification of reptiles during the Triassic and also sheds light on the interrelationships of a wide range of important groups. The authors present a solid phylogenetic analysis of sauropterygians, which reveals a possible new clade, Sauropterygomorpha, which is recovered close to Archosauromorpha. The authors suggest that the latter, as well as the Testudines and Ichthyosauromorpha, belong to a clade that had previously only been recovered using phylogenomic data, the Archelosauria. This paper will be of interest to a broad range of scientists, including palaeontologists, herpetolgists, and evolutionary biologists.
Our editorial process produces two outputs: (i) public reviews designed to be posted alongside the preprint for the benefit of readers; (ii) feedback on the manuscript for the authors, including requests for revisions, shown below. We also include an acceptance summary that explains what the editors found interesting or important about the work.
Decision letter after peer review:
Thank you for submitting your article "An armored marine reptile from the Early Triassic of South China" for consideration by eLife. Your article has been reviewed by 2 peer reviewers, and the evaluation has been overseen by a Reviewing Editor and George Perry as the Senior Editor. The following individual involved in review of your submission has agreed to reveal their identity: Michael Benton (Reviewer #1).
The reviewers have discussed their reviews with one another, and the Reviewing Editor has drafted this to help you prepare a revised submission.
Essential revisions:
As you can see, there was considerable enthusiasm for the work, and there are only a few comments:
Please implement the changes suggested by reviewer 1 (mostly typos and improvements of figure layout etc).
Add a few more comments on the recently published paper "Wei Wang, Qinghua Shang, Long Cheng, Xiao-Chun Wu, Chun Li, Ancestral body plan and adaptive radiation of sauropterygian marine reptiles, iScience, 25(12) 2022, https://doi.org/10.1016/j.isci.2022.105635." (see comments by reviewer 2).
Reviewer #1 (Recommendations for the authors):
There are two presentations on the geology of the locality and fauna lines 121-132 and
170-197 (stratigraphic note). I would combine the Stratigraphic note with the short existing section on Geological setting to avoid repetition and show you deal with the age of the fauna before describing the fossil.
Figure 1: Make sure the lettering is large enough to be read – especially on the stratigraphic chart.
105: different.
278, 280, 285: you refer to plates in Huene (1936) as taf. [=Tafel], but pl. at line 422; I think I'd use 'pl.' in each case, translating into English. Also, I see pl. XIII instead of pl. 13, at line 422; do we use Latin numbers sometimes and Arabic numbers at other times? I'd standardise to Arabic.
329: 1836 = 1936
498: 1836 = 1936
579: linage = lineage
601: spelnial = splenial
762: whch = which
801: monophyletic
Incomplete references: Hirasawa et al. 2013; Jiang et al. 2016; Li and Liu 2020; Moon and Stubbs 2020; Scheyer et al. 2017; Xu et al. 2022 – these all lack article numbers. You need to train Endnote or Procite properly!
Reviewer #2 (Recommendations for the authors):
I know that the publication of Wei Wang, Qinghua Shang, Long Cheng, Xiao-Chun Wu, Chun Li, Ancestral body plan and adaptive radiation of sauropterygian marine reptiles, iScience, 25(12) 2022, https://doi.org/10.1016/j.isci.2022.105635 was only very recently published and it is already cited in the manuscript. However, Wolniewicz et al. have to discuss the phylogenetic relationships found in this paper as well as the implications drawn on ancestral body shape in Wei Wang et al. 2022 in more detail in their manuscript since these authors analyse the same marine reptile groups but came up with different hypotheses. I know that one major problem is the different interpretation of Hanosaurus and its referred specimen and Lariosaurus sanxian. Wolniewicz et al. already included a discussion of the referred specimen of Hanosaurus and justified by morphological arguments their different interpretation. This is all fine but nevertheless the phylogenetic conclusions needs to be compared and discussed/need some elaborations. A good way to do so will be to compare/discuss the charatcers which led to the one and the other result/phylo. interrelationships.
And of course one have always to consider that many taxa included in all these analyses are based (at least partially) only on incomplete single specimens. This is very problematic because we know from other taxa represented by several individuals that marine reptiles are prone to ontogenetic-intraspecific and sexual dimorphism. Thus drawing conclusions and erecting new taxa on the basis of single and incomplete individuals is problematic. However, I understand that this is the way it goes and only new material can verify or falsify conclusions drawn on those specimens.
1. I would suggest to change or make an addendum to the title since your paper indicates more than just another new reptile -and its implications for new phylo hypos or so (lines 1–2).
2. difrerent (line 105).
3. I do not understand this character since most of the early marine reptiles (e.g., Lario sanxian., Hano, Majiash. etc) do NOT have osteoderms (line 150).
4. From your figure it is also possible that this specimen has 4 to 5 sacral vert/ribs, since the rib anterior to your sr1 also is distally expanded as is the rib posterior to your sr3. please explain why these are not sr (line 299).
5. [a rugose dorsolateral surrface] please show/add in figure (line 310).
6. aff Eusaurosphargis from Winterswijk does have uncinate processes on dorsal ribs but maybe this depends on anatomical position and ontogeny ? (line 333)
7. [‘rib-basket’] can you please visualize this by a sketch not totally clear what you mean (line 338).
8. As far as I understand the coracoid is an important element to distinguish taxa please add an overview figure (photo or sketch) of the coracoids of all impoartnat taxa (line 413).
9. Why ‘Lariosaurus’/in quotation marks (line 419).
10. This [a weak connection between dorsal neural arches and centra] is one characteristic of Sauropterygia , summarized in Rieppel 2000; in addition vertebral ossification follows in different groups different patterns, it is thus not necessarily a good indicator for ontogeny (line 549).
11. You must discuss and compare your [phylogenetic] results with those of Wang et al. 2022 (line 560).
12. ploytomy (line 563).
13. Did you also include in another version Cyamodontidae/heavily armored placodonts ? I am wondering if this would make any change (line 570).
14. "small" etc is a difficult character which should be defined (for example give a ratio or smaller than XY) (line 604).
15. Sauopterygomorpha (line 667).
16. Please add reference for this statement (line 676).
17. posteriory (line 677).
18. ? to my knowledge there is no bone histological study on Eusaurosphargis so far; microanatomy based on Ct data are mentioned in Scheyer et al. 2017 (line 679).
19. Unclear statement please rephrase to be clearer (line 704).
20. Please add results and discuss those from Wang et al. 2022 which have a completely different hypo (line 748).
21. demonstreates (line 753).
22. eosauropterygia (line 767).
23. unclear/why (lines 777–778; 792–793).
24. Please shortly discuss some of the characters which lead to this result (line 799).
Essential revisions:
As you can see, there was considerable enthusiasm for the work, and there are only a few comments:
- Please implement the changes suggested by reviewer 1 (mostly typos and improvements of figure layout etc).
Please see responses to Reviewer 1 comments below.
- Add a few more comments on the recently published paper "Wei Wang, Qinghua Shang, Long Cheng, Xiao-Chun Wu, Chun Li, Ancestral body plan and adaptive radiation of sauropterygian marine reptiles, iScience, 25(12) 2022, https://doi.org/10.1016/j.isci.2022.105635." (see comments by reviewer 2).
Please see responses to Reviewer 2 comments below.
Reviewer #1 (Recommendations for the authors):
There are two presentations on the geology of the locality and fauna lines 121-132 and
170-197 (stratigraphic note). I would combine the Stratigraphic note with the short existing section on Geological setting to avoid repetition and show you deal with the age of the fauna before describing the fossil.
The stratigraphic note (lines 170–197) was integrated with the text of the Geological Background section (lines 121–132), in line with the reviewers suggestions.
Figure 1: Make sure the lettering is large enough to be read – especially on the stratigraphic chart.
The font size in Figure 1 was increased according to the reviewers suggestion and a revised Figure 1 was included in the revised version of the manuscript.
105: different.
278, 280, 285: you refer to plates in Huene (1936) as taf. [=Tafel], but pl. at line 422; I think I'd use 'pl.' in each case, translating into English. Also, I see pl. XIII instead of pl. 13, at line 422; do we use Latin numbers sometimes and Arabic numbers at other times? I'd standardise to Arabic.
329: 1836 = 1936
498: 1836 = 1936
579: linage = lineage
601: spelnial = splenial
762: whch = which
801: monophyletic
The references to specific figures and plates were removed from the in-text citations to improve consistency and clarity of the manuscript. We thank the reviewer for correcting the typos. All of them were corrected in the revised manuscript.
Incomplete references: Hirasawa et al. 2013; Jiang et al. 2016; Li and Liu 2020; Moon and Stubbs 2020; Scheyer et al. 2017; Xu et al. 2022 – these all lack article numbers. You need to train Endnote or Procite properly!
We thank the reviewer for pointing out these incomplete references. The article numbers were added to these references in the revised manuscript.
Reviewer #2 (Recommendations for the authors):
I know that the publication of Wei Wang, Qinghua Shang, Long Cheng, Xiao-Chun Wu, Chun Li, Ancestral body plan and adaptive radiation of sauropterygian marine reptiles, iScience, 25(12) 2022, https://doi.org/10.1016/j.isci.2022.105635 was only very recently published and it is already cited in the manuscript. However, Wolniewicz et al. have to discuss the phylogenetic relationships found in this paper as well as the implications drawn on ancestral body shape in Wei Wang et al. 2022 in more detail in their manuscript since these authors analyse the same marine reptile groups but came up with different hypotheses. I know that one major problem is the different interpretation of Hanosaurus and its referred specimen and Lariosaurus sanxian. Wolniewicz et al. already included a discussion of the referred specimen of Hanosaurus and justified by morphological arguments their different interpretation. This is all fine but nevertheless the phylogenetic conclusions needs to be compared and discussed/need some elaborations. A good way to do so will be to compare/discuss the charatcers which led to the one and the other result/phylo. interrelationships.
And of course one have always to consider that many taxa included in all these analyses are based (at least partially) only on incomplete single specimens. This is very problematic because we know from other taxa represented by several individuals that marine reptiles are prone to ontogenetic-intraspecific and sexual dimorphism. Thus drawing conclusions and erecting new taxa on the basis of single and incomplete individuals is problematic. However, I understand that this is the way it goes and only new material can verify or falsify conclusions drawn on those specimens.
Please see detailed responses below.
1. I would suggest to change or make an addendum to the title since your paper indicates more than just another new reptile -and its implications for new phylo hypos or so (lines 1–2).
We thank the reviewer for this suggestion. The title of the manuscript was modified into “An armoured marine reptile from the Early Triassic of South China and its phylogenetic and evolutionary implications”.
2. difrerent (line 105).
Corrected to ‘different’ (typo also noticed by Reviewer 1).
3. I do not understand this character since most of the early marine reptiles (e.g., Lario sanxian., Hano, Majiash. etc) do NOT have osteoderms (line 150).
Establishing the presence of osteoderms as a synapomorphy of Sauropterygomorpha (defined as the last common ancestor of Eusaurosphargis + Pistosaurus and all of its descendants) results from our phylogenetic analysis in TNT 1.5, which reconstructed the presence of osteoderms as a synapomorphy of the Sauropterygomorpha node. This is why this character state is included in the diagnosis of the clade. Please note that Eusaurosphargis, Placodus, Saurosphargidae and Pomolispondylus – the most basal member of the lineage leading to Eosauropterygia according to our phylogenetic hypothesis – all share the presence of osteoderms, supporting it as a synapomorphy of Sauropterygomorpha (osteoderms were likely convergently lost in Atopodentatus, Paraplacodus and the lineage leading to Eosauropterygia crownward of Pomolispondylus).
4. From your figure it is also possible that this specimen has 4 to 5 sacral vert/ribs, since the rib anterior to your sr1 also is distally expanded as is the rib posterior to your sr3. please explain why these are not sr (line 299).
In our judgment, the rib lying immediately posterior to ‘sr3’ on the left side of HFUT YZSB-19-109 does not have an expanded distal end and closely matches the first caudal rib of Largocephalosaurus in morphology (compare with Li et al. 2014 [GeolMag]:Figures4i, 5d and 6a), so we accordingly identify it as a caudal rib. However, we agree with Reviewer 2 that the exact number of sacral ribs/vertebrae is difficult to establish in the new taxon, because the posterior dorsal/sacral ribs are partially obscured by the overlying ilium and ischium. The difficulties of establishing the correct number of sacral ribs were added to the manuscript (lines 315–320):
”Four complete posterior dorsal/sacral vertebrae are preserved and exposed in left ventrolateral view. Two sacral vertebrae can be identified by the presence of associated sacral ribs with clearly expanded distal ends. However, the distal ends of the first two ribs lying immediately posterior to the last unambiguous dorsal rib are obscured by the overlying ilium and ischium, so it is not possible to confidently determine whether they represent the last two dorsal ribs or the first two sacral ribs (Figure 6A).”
All of the parts of the manuscript that mentioned the number of sacral vertebrae/ribs were updated in accordance with this anatomical revision.
5. [a rugose dorsolateral surrface] please show/add in figure (line 310).
A label for the rugose dorsal surface of the neural arch (‘rgs’) was added to Figure 6B.
6. aff Eusaurosphargis from Winterswijk does have uncinate processes on dorsal ribs but maybe this depends on anatomical position and ontogeny ? (line 333)
It IS stated here that the dorsal ribs of Eusaurosphargis DO possess uncinate processes and thus differ in this respect from the ribs of HFUT YZSB-19-109, in which uncinate processes are absent.
7. [‘rib-basket’] can you please visualize this by a sketch not totally clear what you mean (line 338).
The ‘rib-basket’ is a saurosphargid synapomorphy introduced by Li et al. (2011:309):
“Distally, the [dorsal] rib quickly expands in its anteroposterior dimension to a degree that results in a contact of the successive ribs with one another (Figure 3B). Collectively, the ribs lying parallel to each other form a closed dorsal ‘rib basket.’”
An appropriate explanation was provided alongside the first appearance of the term ‘rib-basket’ in the main text (line 84):
“broadened dorsal ribs forming a closed rib-basket”.
The term was also replaced by ‘armoured’ in the abstract, to improve clarity for the broader readership.
8. As far as I understand the coracoid is an important element to distinguish taxa please add an overview figure (photo or sketch) of the coracoids of all impoartnat taxa (line 413).
A new figure presenting the coracoid morphology of selected Early–Middle Triassic sauropterygomorph taxa (Figure 7 of the revised manuscript) was added to the manuscript and referred to throughout the text.
9. Why ‘Lariosaurus’/in quotation marks (line 419).
As noted by Wang et al. (2022 [iScience]:2–4), Lariosaurus sanxiaensis differs from typical representatives of the genus Lariosaurus in several anatomical features (most notably in having a sub-oval rather than an anteroposteriorly constricted coracoid) and stratigraphic occurrence (Lariosaurus is otherwise known exclusively from the Middle Triassic), raisinig the possibility that Lariosaurus sanxiaensis represents a different genus. As a consequence, we used quotation marks for ‘_Lariosaurus_’ sanxiaensis in the previous version of the manuscript to indicate its probable taxonomic distinctness. However, neither Lariosaurus sanxiaensis nor any Middle Triassic representative of Lariosaurus was included in our phylogenetic analysis, and the holotype and referred specimens of Lariosaurus sanxiaensis were not studied during the preparation of this manuscript, so we decided to remove the quotation marks in the revised version of manuscript and refrain from discussing the taxonomy of Lariosaurus sanxiaensis in the current study.
10. This [a weak connection between dorsal neural arches and centra] is one characteristic of Sauropterygia , summarized in Rieppel 2000; in addition vertebral ossification follows in different groups different patterns, it is thus not necessarily a good indicator for ontogeny (line 549).
We thank the reviewer for pointing this out. Following the reviewer’s suggestion, the information about a weak connection between the neural arch and centrum was removed from the Ontogeny and body size section and included in the description of the vertebrae, where the feature was more appropirately referred to as a sauropterygian synapomorphy. Furthermore, the ontogeny and body size section was removed from the manuscript altogether and the text on body size estimation was moved to the first paragraph of the anatomical description (lines 311–314):
“Articulated anterior and posterior dorsal neural arches preserved without their respective centra indicate a lack of fusion of the neurocentral suture, a paedomorphic feature characteristic for Sauropterygia (Rieppel 2000a) and also present in Saurosphargis (Huene 1936) and Largocephalosaurus (Li et al. 2014).”
11. You must discuss and compare your [phylogenetic] results with those of Wang et al. 2022 (line 560).
Following the reviewer’s suggestion, the following paragraphs explaining the major differences between the phylogenetic hypotheses of Wang et al. (2022) and the one presented in this manuscript were added to the Phylogeny of Sauropterygomorpha section of the Discussion:
“The results of our phylogenetic analysis differ significantly from the results of a phylogenetic analysis recently published by Wang et al. (2022), in which Hanosaurus was recovered as the sister-group to a clade comprising Saurosphargidae + Sauropterygia within a monophyletic Sauropterygiformes. However, we believe that our phylogenetic analysis presents a more accurate topology of sauropterygians and their relatives for the following reasons. Wang et al. (2022) used 16 outgroup (non-sauropterygiform) taxa (15 marine reptiles and a single terrestrial reptile), representing five major reptile lineages, whereas our study included 40 outgroup (non-sauropterygomorph) taxa (19 terrestrial and 21 aquatic reptiles), representing 23 major reptile lineages. The inclusion of only a single terrestrial outgroup taxon – Youngina – in the analysis of Wang et al. (2022) is problematic because Youngina likely represents a taxon rather distantly related to the Mesozoic marine reptile clade which includes Sauropterygia (Figure 8; see also Simões et al. 2022). Distantly related taxa likely share fewer character states with derived taxa (homoplasy accumulation through time), so a phylogenetic analysis containing a single, distantly related outgroup taxon will likely fail to adequately capture important character transformation sequences, in contrast to a phylogenetic analysis in which outgroups are more comprehensively sampled (Wilberg 2015). Furthermore, the phylogenetic analysis of Wang et al. (2022) used 181 morphological characters, in contrast to 221 characters included in our study. Greater character sampling has been demonstrated as an important factor increasing the accuracy of phylogenetic reconstructions (Wiens 2006), which also favours the results obtained by our analysis.
The different topologies recovered by both studies are likely also partially a consequence of differences in character scoring. For example, Wang et al. (2022) scored the humerus of Hanosaurus as ‘rather straight’ (plesiomorphic state), similar to the humerus of Youngina and other non-sauropterygiform marine reptiles. However, in our opinion, the humerus morphology of Hanosaurus matches the typical ‘curved’ morphology (derived state) characteristic of saurosphargids, placodonts and the majority of eosauropterygians (Rieppel 2000a). Wang et al. (2022) also scored Hanosaurus into an updated version of the phylogenetic matrix of Neenan et al. (2013). Hanosaurus was recovered as the earliest-diverging sauropterygiform in this analysis as well, but its humerus was also scored as plesiomorphic (‘rather straight’) in the character-taxon matrix. Interestingly, this updated analysis of Neenan et al. (2013) included a more comprehensive outgroup (non-sauropterygiform) sample (12 terrestrial and 5 aquatic taxa, representing 12 major reptile lineages) than the analysis of Wang et al. (2022) and recovered Eusaurosphargis and Helveticosaurus as successive sister-groups to Sauropterygia – a result similar to the one obtained in this study (Figure 9).”
We also referred to another recently published paper on the phylogenetic interrelationships among reptiles (Simões et al. 2022 [SciAdv]) throughout the text.
12. ploytomy (line 563).
Corrected to ‘polytomy’.
13. Did you also include in another version Cyamodontidae/heavily armored placodonts ? I am wondering if this would make any change (line 570).
The phylogenetic matrix used in this study was originally constructed to include early-diverging representatives of various reptile groups. However, the morphology of cyamodontoid placodonts is very derived in comparison with their early-diverging members (modified skull, dentition, girdles, body armour). Some of these derived features (such as the anteroposteriorly short premaxilla) are convergent with similar features seen in early-diverging sauropterygomorphs (anteroposteriorly short premaxilla in Eusaurosphargis), which might bias the results of the phylogenetic analysis presented in this manuscript if cyamodontoid placodonts were included. To prevent some of these convergent character states from influencing the results of our analyses, the inclusion of cyamodontoid placodonts into our phylogenetic matrix would also necessitate the addition/revision of several characters and character states, which is beyond the scope of the present study. We therefore do not include cyamodontoid placodonts in the current study, and consider them as derived taxa of limited relevance for addressing our specific research questions.
14. "small" etc is a difficult character which should be defined (for example give a ratio or smaller than XY) (line 604).
A statement explaining that in this context ‘small’ means anteroposteriorly short, together with an appropriate reference containing definitions of both character states used to describe the relative size of the premaxilla (Chen et al. 2014 PloS One), were added to the revised version of the manuscript (lines 674–678).
15. Sauopterygomorpha (line 667).
Corrected to ‘Sauropterygomorpha’.
16. Please add reference for this statement (line 676).
Appropriate reference is already cited at the end of the sentence:
“Both of these character states are considered as typical for terrestrial taxa and contrast with the enlarged premaxillae and posteriory displaced external narial openings characteristic for marine reptiles (Chen et al. 2014a).”
17. posteriory (line 677).
Corrected into ‘posteriorly’.
18. ? to my knowledge there is no bone histological study on Eusaurosphargis so far; microanatomy based on Ct data are mentioned in Scheyer et al. 2017 (line 679).
Replaced ‘histology’ with ‘microanatomy’.
19. Unclear statement please rephrase to be clearer (line 704).
Replaced ‘placodonts’ with ‘placodont fossils’ for clarity.
20. Please add results and discuss those from Wang et al. 2022 which have a completely different hypo (line 748).
Please see response to comment #11.
21. demonstreates (line 753).
Corrected into ‘demonstrates’.
22. eosauropterygia (line 767).
Sentence containing typo was deleted as it contained a factual error.
23. unclear/why (lines 777–778; 792–793).
Lines 777–778 and 792–793 were edited in order to more clearly explain that the dermal armour present in early sauropterygian ancestors most likely countercated buoyanacy and enabled these animals to explore the bottom of the shallow sea in search of food:
“Our phylogenetic analysis indicates the important role of body armour in sauropterygian evolution (Figure 10). Dermal armour was likely an important preadaptation that allowed colonisation of the shallow marine realm by a _Eusaurosphargis_-like ancestor, enabling it to counteract buoyancy and walk on the bottom of the shallow sea in search of food (Houssaye 2009). Elaboration of dermal armour occurred in the shallow marine placodonts and saurosphargids, perhaps as a response to predation pressure (Liu et al. 2014; Chen et al. 2014b; Qiao et al. 2020). The reduction and complete loss of dermal armour then occurred in the lineage leading to Eosauropterygia, and was likely involved with the evolution of active predation, an efficient swimming style and increasing adaptation to a pelagic lifestyle.”
“These evolutionary parallels seem to demonstrate that dermal body armour could have been a possible prerequsite (preadaptation) for the invasion of the shallow marine realm in different diapsid clades, which allowed for buoyancy reduction and exploration of the shallow marine environment in search of food.”
24. Please shortly discuss some of the characters which lead to this result (line 799).
The synapomorphies of Archelosauria have already been listed in the Phylogenetic Results section and the following brief discussion was added to the end of the final paragraph of the Discussion section:
“Archelosauria are supported in our analysis by four unambiguous synapomorphies associated with the morphology of the skull and shoulder girdle (see above). This demonstrates that innovations of the cranium linked with the evolution of sensory organs and the feeding apparatus, as well as changes in locomotion, perhaps underlie the evolutionary success of archelosaurian reptiles.”
Supplementary Materials
MDAR checklist
Source data 1. Character-taxon matrix used in the phylogenetic analysis.
Data Availability Statement
Specimen HFUT YZSB-19-109 is housed in the collections of the Geological Museum, Hefei University of Technology, Hefei, China and available for examination upon request to JL. The phylogenetic data matrix used in this study is available in Source data 1.