Mei-P26 regulates microRNAs and cell growth in the Drosophila ovarian stem cell lineage (original) (raw)
. Author manuscript; available in PMC: 2010 Nov 19.
Published in final edited form as: Nature. 2008 Jun 4;454(7201):241–245. doi: 10.1038/nature07014
Abstract
Drosophila neuroblasts1 and ovarian stem cells2,3 are well characterized models for stem cell biology. In both cell types, one daughter cell self-renews continuously while the other undergoes a limited number of divisions, stops to proliferate mitotically and differentiates. Whereas neuroblasts segregate the Trim–NHL (tripartite motif and Ncl-1, HT2A and Lin-41 domain)-containing protein Brain tumour (Brat) into one of the two daughter cells4-6, ovarian stem cells are regulated by an extracellular signal from the surrounding stem cell niche. After division, one daughter cell looses niche contact. It undergoes 4 transit-amplifying divisions to form a cyst of 16 interconnected cells that reduce their rate of growth and stop to proliferate mitotically. Here we show that the Trim–NHL protein Mei-P26 (refs 7, 8) restricts growth and proliferation in the ovarian stem cell lineage. Mei-P26 expression is low in stem cells but is strongly induced in 16-cell cysts. In mei-P26 mutants, transit-amplifying cells are larger and proliferate indefinitely leading to the formation of an ovarian tumour. Like brat, mei-P26 regulates nucleolar size and can induce differentiation in Drosophila neuroblasts, suggesting that these genes act through the same pathway. We identify Argonaute-1, a component of the RISC complex, as a common binding partner of Brat and Mei-P26, and show that Mei-P26 acts by inhibiting the microRNA pathway. Mei-P26 and Brat have a similar domain composition that is also found in other tumour suppressors and might be a defining property of a new family of microRNA regulators that act specifically in stem cell lineages.
When Drosophila germline stem cells divide (Fig. 1a), 1 daughter cell (the cystoblast) looses niche contact and undergoes 4 transit-amplifying divisions to create a cyst of 16 cystocytes, which remain connected by the fusome. In each cyst, 1 cell becomes the oocyte, whereas the other cells undergo endoreplication to form 15 so-called nurse cells.
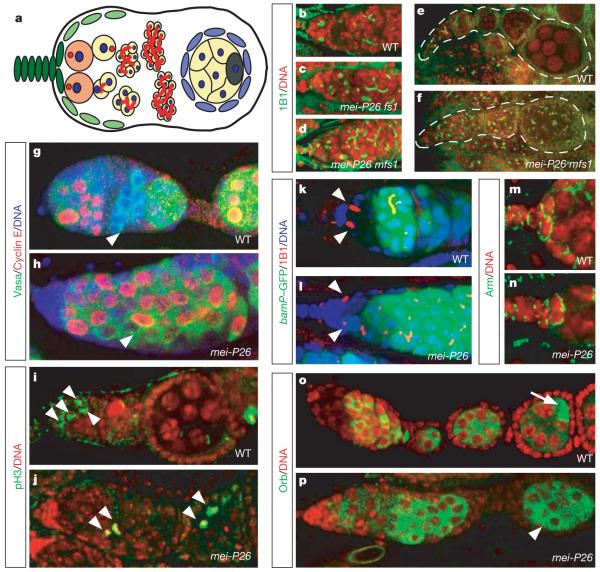
Figure 1. Differentiation and cell cycle defects in mei-P26 mutant ovaries.
a, Overview of Drosophila oogenesis showing cap cells (dark green), escort cells (light green), stem cells (orange), cystoblasts and cystocytes (yellow), follicle cells (blue) and the oocyte (dark grey). The spectrosome and fusome are red. b–f, Wild type (WT) (b, e) and mei-P26 (c, d, f) ovarioles stained with mAb1B1 (1B1, green) and for DNA (red). fs1 and mfs1 represent two different mei-P26 alleles. g, h, Cyclin E (red; green, Vasa; blue, DNA) is downregulated in WT (g, arrowhead) but not in the mei-P26fs1 mutant (h, arrowhead) cystocytes. i, j, Phospho-H3 positive (green, pH3; red, DNA) mitotic cells (arrowheads) are restricted to the tip of the ovarioles in WT (i) but are found at all stages in mei-P26fs1 (j) ovarioles. k, l, GFP under the control of the bam promoter (bamP–GFP) (green; red, mAb1B1; blue, DNA) is not expressed in WT (k) and mei-P26fs1 mutant (l) niche contacting germline cells (arrowheads). m, n, Anti-Armadillo (green; red, DNA) shows integrity of WT (m) and mei-P26fs1 (n) mutant cap cells. o, p, Orb expression is initiated in WT (o) and mei-P26fs1 mutant (p) cystocytes but restricted to the oocyte only in WT (o, arrow) and not in mei-P26fs1/mfs1 mutant (p, arrowhead) egg chambers.
Because brat192 mutant germline clones do not show any obvious defects in oogenesis (data not shown), we analysed the ovarian phenotype of mei-P26. Like Brat, Mei-P26 is a Trim–NHL protein9 and carries an NHL domain, a coiled-coil region and several B-boxes. Weak mei-P26 mutants have defects in meiosis7 whereas stronger alleles cause tumourous overproliferation8. mei-P26 mutant ovaries were stained using the monoclonal antibody mAb1B1 (ref. 10), which labels the fusome and the spectrosome—a cytoplasmic organelle only present in stem cells and cystoblasts11 (Fig. 1b–f). Wild-type ovaries contain two stem cells near the tip of the germarium whereas cystoblasts and cysts are separated from the stem cell niche (Fig. 1b). In mei-P26 mutant ovaries, germaria are filled with individual spectrosome-containing cells (19%) and cysts containing varying numbers of cells (<4 in 39%, >4 in 42%; n > 400 cells) connected by a fusome (Fig. 1c, d and Supplementary Fig. 1a, a’). Nurse cells and oocytes are not formed, and fusomes or spectrosomes are maintained at later stages of oogenesis (Fig. 1e, f). In wild-type ovaries, the S-phase marker Cyclin E oscillates with the cell cycle in stem cells and mitotically active cysts, is downregulated as cystocytes exit mitotic proliferation and is re-expressed as nurse cells enter endoreplication12 (Fig. 1g). In mei-P26 mutant ovaries, however, Cyclin E is highly expressed at all stages of oogenesis (Fig. 1h). Upregulation of Cyclin E occurs even in small mei-P26 clones (Supplementary Fig. 1b, c), and is therefore not an indirect consequence of tumour formation. Phospho-histone-H3-positive mitotic germline cells are restricted to the anterior tip of the germarium in wild-type ovaries (Fig. 1i) but are detected throughout the ovarioles in mei-P26 mutants (Fig. 1j and Supplementary Fig. 1a, a’). Whereas all cells in a wild-type cyst divide synchronously, mei-P26 mutant cysts frequently contain both mitotic and interphase cells (data not shown). Thus, mei-P26 is required for proliferation control and differentiation in the female germline stem cell lineage.
To test stem cell niche signalling, we used a green fluorescent protein (GFP) fusion to the bag of marbles (bam) promoter13, which is suppressed by Decapentaplegic (Dpp) from the niche in stem cells but not in cystocytes (Fig. 1k and Supplementary Fig. 1d). In mei-P26 mutants (Fig. 1l and Supplementary Fig. 1e), bam transcription is repressed in niche-contacting cells but is upregulated in cystocytes. Because staining for the niche marker Armadillo (Fig. 1m, n) reveals no structural abnormalities and because ovarian tumours are also observed when mei-P26 is removed exclusively from the female germ line (Supplementary Fig. 1f, g), mei-P26 is not required for sending or receiving the niche signal.
mei-P26 mutant tumour cells have branched fusomes and express Bam. To confirm further that these cells do not have stem cell identity, we analysed the expression of oo18 RNA-binding protein (Orb). Orb expression starts in all cystocytes between the 8- and 16-cell stage14, but is restricted to the oocyte during later stages (Fig. 1o). In mei-P26 mutants, Orb expression is initiated normally (Fig. 1p) and is detected throughout the tumour. However, Orb is never restricted to a single cell, suggesting that the oocyte is not specified. Thus, mei-P26 mutants develop a ‘cystocytoma’ in which tumour cells express markers for the cystocyte fate. Single-spectrosome-containing cells in the tumour might arise from occasional disintegration of fusome-connected cysts, a process that has been described before15.
Brat can inhibit cell growth and ribosome biogenesis16. mei-P26 might also regulate cell growth because overexpression from the eyeless promoter reduces eye size (Supplementary Fig. 2a, b), even when cell death is inhibited by co-expressing p35 (data not shown). To test whether cell growth is differentially regulated in the ovarian stem cell lineage, we quantified the volume of stem cells and cysts after three-dimensional reconstruction (see Methods). Shortly after stem cell division (elongated spectrosome morphology), stem cells are 314 ± 13 μm3 (mean ± s.e.m.; n = 4) whereas cystoblasts are 330 ± 30 μm3 (n = 4). Stem cells grow to a maximum of 600 μm3 (average 437 ± 21 μm3, n = 19) and double their volume between each mitotic division (about once per day). To measure cyst volumes, mitotic clones were marked by the absence of GFP (Fig. 2a). Three- and four-day-old 16-cell cysts are 1,215 ± 61 μm3 (n = 7) and 1,163 ± 48 μm3 (n = 7), respectively. Thus, cell growth slows down as cells exit mitotic proliferation. Consistent with this, cystocytes become progressively smaller during transit-amplifying divisions (Fig. 2g). diminutive (dMyc), the Drosophila Myc homologue, an important regulator of cell growth, is highly expressed in stem cells and cystoblasts but downregulated in 16-cell cysts (Fig. 2e). Nucleoli (stained by anti-Fibrillarin)—the sites of ribosomal RNA transcription—are large in stem cells but much smaller in 16-cell cysts (Fig. 2c and Supplementary Fig. 2c). Because ribosome number is thought to control cellular growth in Drosophila17,18, a reduction in ribosome biogenesis might be responsible for the reduced cell growth at the end of mitotic proliferation.
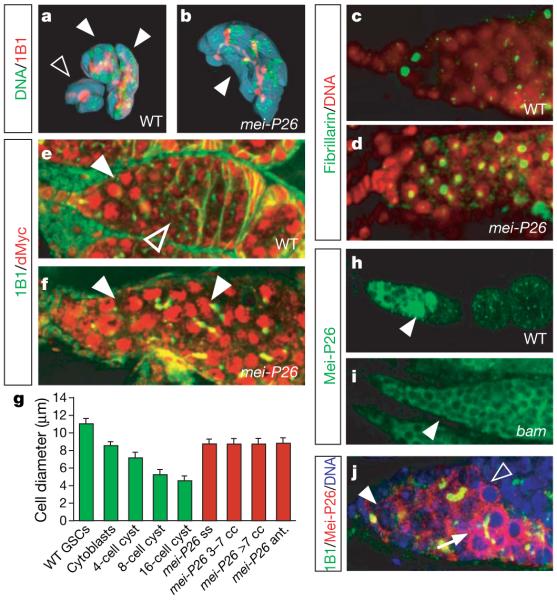
Figure 2. Mei-P26 regulates cell and nucleolar size.
a, b, Three-dimensional reconstruction of WT 16-cell cysts (filled arrowheads; a), a WT 8-cell cyst (open arrowhead; a) and a mei-P26fs1/mfs1 mutant cyst containing 14 cells (b). c, d, Nucleoli (green, anti-Fibrillarin) in mei-P26fs1 mutant ovaries (d) are larger than those in the WT ovaries (c). e, f, High levels of dMyc (red; green, mAb1B1) are detected in germline stem cells and early cysts (filled arrowhead). In postmitotic cysts, dMyc levels are decreased (open arrowhead) and levels increase again as nurse cells undergo endoreplication (e). In mei-P26mfs1/fs1 mutants, high levels of dMyc are detected throughout the tumour (arrowheads in f). g, Diameter of the indicated cell types in WT (n > 24 cells) and mei-P26fs1/mfs1 mutant (n = 11 cells for Mei-P26 ant., n > 43 for all others) ovaries. Abbreviations: Mei-P26 ss, single-spectrosome-containing cells; Mei-P26 3–7 cc (or Mei-P26 >7 cc), cystocytes in mei-P26fs1 mutant cysts containing either 3–7 (or >7) cells; Mei-P26 ant., anterior niche contacting cells. Error bars, s.e.m. (green bars, WT; red bars, mei-P26 mutant). h, i, Mei-P26 expression peaks in early 16-cell cysts in WT and is not detected at later stages of oogenesis (h). In bamΔ86 mutant ovaries, expression is not upregulated (i, compare to WT in h). Arrowheads point at equivalent stages. Note that Mei-P26 staining appears more intense in later stages owing to sample thickness and out-offocus fluorescence (i). j, Germarium close up: Mei-P26 (red; green, mAb1b1; blue, DNA) expression is low in stem cells (arrowhead), weakly upregulated in 8-cell cysts (open arrowhead) and peaks in 16-cell cystocytes (arrow) (see Supplementary Fig. 2e, f, h).
In mei-P26 mutants, cellular and nucleolar size are increased and dMyc is highly expressed throughout the tumour (Fig. 2d, f, g and Supplementary Fig. 2c): the volume of 16-cell cysts is increased to 3,956 ± 424 μm3 (n = 4; Fig. 2b) and cell diameters no longer decrease as cells are displaced from the stem cell niche (Fig. 2g). Thus, mei-P26 is responsible for the differential regulation of cell growth in the Drosophila ovarian stem cell lineage. Like brat, it might achieve its function by regulating ribosome biogenesis and controlling the expression of dMyc.
To analyse Mei-P26 expression, we generated a specific antibody (Supplementary Fig. 2d–f). Mei-P26 mRNA and protein levels are low in stem cells but are upregulated in cysts as they decrease growth and exit mitotic proliferation (Fig. 2h, j and Supplementary Fig. 2e, g, h). This regulation is functionally important because mei-P26 overexpression in ovaries results in stem cell loss and complete depletion of the female germ line (Fig. 3a, b). In bam mutant ovaries, Mei-P26 levels remain low suggesting that mei-P26 is regulated in a Bam-dependent manner (Fig. 2i). bam overexpression induces premature differentiation of stem cells in a wild-type (Fig. 3d) but not in a mei-P26 mutant (Fig. 3e) background, demonstrating that Mei-P26 is essential for Bam to induce cystocyte differentiation. Consistently, germline stem cells do not differentiate when pumilio is removed in a mei-P26 mutant background (Supplementary Fig. 2k, l). To test whether Mei-P26 is the only target of Bam, we overexpressed mei-P26 in a bam mutant background (Fig. 3f, g and Supplementary Fig. 2i, j). mei-P26 overexpression reduces the size of bam mutant cells (from 10.1 ± 0.17 μm (bam, n = 27) to 7.0 ± 0.19 μm (bam, nanos gal4  UASP-mei-P26, n = 27)) and the enlarged nucleolus in bam mutants (Supplementary Fig. 2c) but does not rescue the differentiation defects (Supplementary Fig. 2i, j) observed in these mutants. Thus, mei-P26 is upregulated by Bam activity in cystocytes and inhibits cell growth and mitotic proliferation.
UASP-mei-P26, n = 27)) and the enlarged nucleolus in bam mutants (Supplementary Fig. 2c) but does not rescue the differentiation defects (Supplementary Fig. 2i, j) observed in these mutants. Thus, mei-P26 is upregulated by Bam activity in cystocytes and inhibits cell growth and mitotic proliferation.
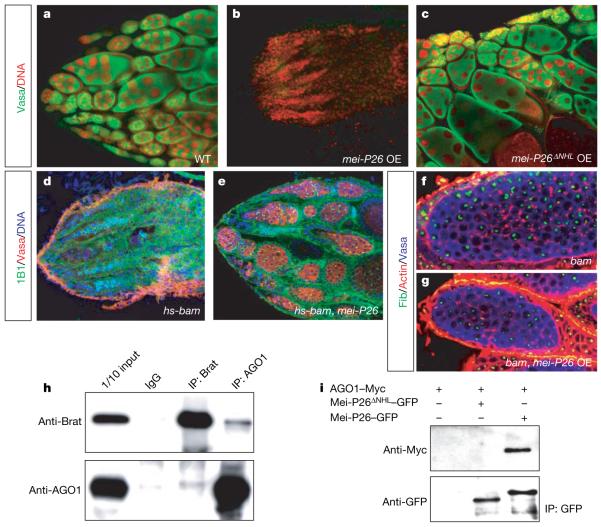
Figure 3. Bam requires the AGO1-binding protein Mei-P26 to induce proper cystocyte differentiation.
a, b, c, mei-P26 (b) but not mei-P26ΔNHL (c) overexpression (OE) (from nanos-Gal4::VP16) depletes the germ line (green, Vasa; red, DNA). d, e, Transient (heat-shock-induced) bam overexpression induces stem cell differentiation and depletes the germ line (red, Vasa; green, mAb1B1; blue, DNA) in a WT (d) but not mei-P26fs1/mfs1 mutant (e) background. f, g, mei-P26 overexpression (from nanos-Gal4::VP16)in bamΔ86 mutants (g) reduces cell and nucleolar size (see statistics in Supplementary Fig. 2c) but does not induce stem cell differentiation (Supplementary Fig. 2i, j). h, i, The NHL domain proteins Brat, Mei-P26 and Dappled interact with AGO1. Reciprocal immunoprecipitations (IP) of Brat and AGO1 from Drosophila embryo extracts (h). GFP-tagged Mei-P26 but not GFP-tagged Mei-P26ΔNHL (i) coimmunoprecipitates Myc-tagged AGO1 in S2 cells (see Supplementary Fig. 4b).
In the brain, mei-P26 is weakly expressed in neuroblasts, is absent from ganglion mother cells but is highly expressed in neurons (Supplementary Fig. 3a–d). Although mei-P26 mutants have no obvious defects in neurogenesis, mei-P26 overexpression can induce premature neuronal differentiation (Supplementary Fig. 3e, f), suggesting that mei-P26 and brat might have some common targets. Using mass spectrometry, we identified the RNase Argonaute-1 (AGO1) in anti-Brat immunoprecipitates (Supplementary Fig. 4a). Brat and AGO1 can be co-immunoprecipitated from Drosophila embryos (Fig. 3h), and co-transfection experiments in S2 cells show that AGO1 also binds Mei-P26 and the Trim-NHL protein Dappled (Supplementary Fig. 4b). This interaction is functionally significant because Mei-P26 lacking the NHL domain no longer binds AGO1 (Fig. 3i) and fails to block self renewal when overexpressed in ovarian stem cells (Fig. 3c).
AGO1 is a core component of the RISC complex19 and is important for microRNA-mediated translational repression and RNA degradation. MicroRNAs are essential for self renewal in Drosophila ovarian stem cells because mutations in AGO1 (Supplementary Fig. 5), dicer-1 (ref. 20) or its binding partner loquacious21 result in premature stem cell differentiation. To test whether Mei-P26 regulates microRNAs, we measured microRNA levels by quantitative reverse transcription polymerase chain reaction (RT–PCR). In mei-P26 mutants, most microRNAs are significantly upregulated (Supplementary Fig. 7a, b), whereas overexpression of mei-P26 in bam mutants broadly reduces microRNA levels (Fig. 4a and Supplementary Fig. 7a). Importantly, the mei-P26 mutant phenotype can be partially rescued by removing one copy of Loquacious and thereby reducing microRNA levels (Fig. 4b, c and Supplementary Fig. 6a–f), indicating that mei-P26 acts by inhibiting the microRNA pathway in cystocytes.
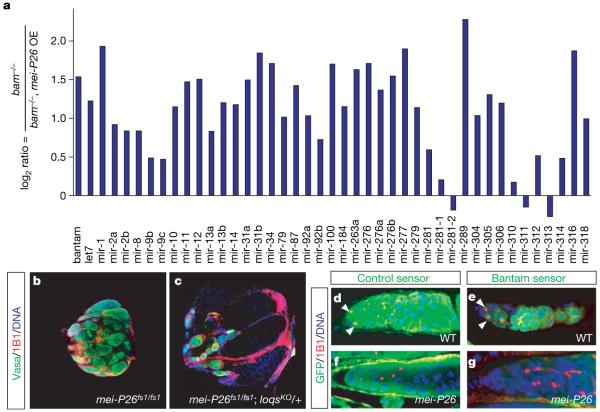
Figure 4. Mei-P26 regulates miRNAs.
a, Quantitative PCR experiment comparing the level of mature microRNAs in bamΔ86 and bamΔ86, nanos-Gal4::VP16 pUASp-mei-P26. The y axis shows log2 of the expression ratio between the two genotypes. b, c, Loss of one copy of loquacious partially rescues the mei-P26fs1 mutant phenotype (Supplementary Fig. 6). d–g, Downregulation of the bantam sensor reveals bantam microRNA expression in stem cells but not cystocytes (e), whereas the control sensor is uniformly expressed throughout the germ line (d). The bantam sensor (g) but not the control sensor (f) is silenced in the germ line of mei-P26fs1/mfs1 mutants (arrowheads indicate stem cells).
pUASp-mei-P26. The y axis shows log2 of the expression ratio between the two genotypes. b, c, Loss of one copy of loquacious partially rescues the mei-P26fs1 mutant phenotype (Supplementary Fig. 6). d–g, Downregulation of the bantam sensor reveals bantam microRNA expression in stem cells but not cystocytes (e), whereas the control sensor is uniformly expressed throughout the germ line (d). The bantam sensor (g) but not the control sensor (f) is silenced in the germ line of mei-P26fs1/mfs1 mutants (arrowheads indicate stem cells).
One of the best characterized Drosophila microRNAs is bantam, a regulator of proliferation and apoptosis22. We used a sensor that expresses GFP from the tubulin promotor and carries two bantam binding sites in the 3′ untranslated region22. In wild-type ovaries, this sensor is repressed by bantam activity in stem cells but is derepressed in 16-cell cystocytes in which high levels of Mei-P26 are present (Fig. 4e). In mei-P26 mutants, the sensor is off in all germline cells (Fig. 4g). In contrast, a control sensor lacking microRNA-binding sites shows high expression in all wild-type and mutant cells (Fig. 4d, f). Although mei-P26 regulates many microRNAs, bantam seems to be an important target: even animals heterozygous for a bantam null mutation have a reduced number of stem cells (1.15 ± 0.12 per germarium (n = 32 germaria) compared to 2.09 ± 0.05 (n = 32 germaria) in 14-day-old bantam heterozygous and wild-type flies, respectively), suggesting a defect in self renewal (Supplementary Fig. 7e, f). To test whether mei-P26 regulates bantam directly, we expressed a luciferase construct carrying a _bantam_-binding site in its 3′ untranslated region in S2 cells. In control cells, the bantam sensor is repressed, but a construct lacking the binding site is not affected (Supplementary Fig. 7d). On cotransfection of mei-P26 (Supplementary Fig. 7c), luciferase expression is significantly derepressed, but no derepression is seen with cotransfection of mei-P26 lacking the NHL domain; this indicates that Mei-P26 can repress bantam activity even in S2 cells.
Our data suggest that brat and mei-P26 might act in a similar manner to control proliferation in stem cell lineages. In both mutants, cells that normally stop self renewal increase ribosome biogenesis, grow abnormally large and fail to exit the cell cycle leading to the formation of a tumour. The general upregulation of microRNAs in mei-P26 mutants leaves several possibilities for how these proteins might regulate the microRNA pathway. The presence of a RING finger in Mei-P26 suggests a role in protein degradation. The high amounts of AGO1 detected in Mei-P26 immunoprecipitates make it unlikely that AGO1 itself is degraded by Mei-P26. However, another member of the RISC complex might be a degradation target of Mei-P26. Equally likely, Mei-P26 could prevent the incorporation or increase the turnover of microRNAs in the RISC complex.
Many human tumours contain cancer stem cells that drive tumour growth and metastasis23,24. Although the similarities between Drosophila tumours and human cancer are limited, brat mutant brains and mei-P26 mutant ovaries (as well as the other mutant conditions causing stem cell tumours25-27) provide an invertebrate model for stem-cell-derived tumour formation. In mei-P26 mutants, tumours originate from cystocytes, the transit-amplifying pool of the ovarian stem cell lineage. In mei-P26 mutants, these cells re-gain the ability to self-renew: after bam overexpression—which leads to premature differentiation of stem cells—the germ line is depleted in a wild-type but not in a mei-P26 mutant background. Thus, mei-P26 tumours arise from growth defects in the transit-amplifying compartment of the ovarian stem cell lineage—a mechanism that could occur in human tumours as well.
Our data establish Trim–NHL proteins as regulators of stem cell proliferation. Vertebrate members of this family exist and are downregulated in human cancer cell lines28 suggesting that their tumour-suppressor function might be conserved in vertebrates as well.
METHODS SUMMARY
For immunofluorescence, ovaries were dissected in PBS, fixed for 20 min in _n_-heptane/PBS (1:1) with 5% PFA, washed three times in PBS containing 0.1% Triton X-100, and stained using standard procedures. Cell volumes were determined after three-dimensional reconstruction from stacks of 0.4-μm sections using IMARIS software. A Rho1–GFP trap (ZCL1957) was used to outline stem cells. To outline individual cysts, mitotic clones, generated by heat-induced expression of FLP recombinase (hs-FLP) and marked by the absence of GFP, were stained with rhodamine-phalloidine. Protein lysates were prepared from 20 ovaries dissected in PBS and extracted with Laemmli buffer, and were run on 10% SDS-polyacrylamide gels. Overexpression constructs were created by amplifying mei-P26 full length, _mei-P26_ΔNHL, AGO1, dappled and brat coding sequences by PCR from total complementary DNA or expressed sequence tags (ESTs) with appropriate primers containing bacterial attachment (attB) sites to mediate intermolecular recombination for Gateway cloning (Invitrogen) or appropriate restriction sites. MicroRNAs were quantified using the TaqMan microRNA assay and a quantitative PCR machine (Applied Biosystems) with primer sets obtained from Applied Biosystems on 10 ng total RNA extracted from ovaries with TRIzol Reagent (Invitrogen). To measure bantam activity, S2 cells were transfected with a plasmid that expresses the yeast transcription factor Gal4 under the control of the actin promoter (actin-Gal4), a control construct expressing Renilla luciferase, a sensor carrying two copies of a sequence complementary to the bantam miRNA downstream of firefly luciferase (FLuc) and the respective pUASp-Mei-P26 constructs. All transfections were performed in triplicate and dual luciferase assays were performed 24 h after transfection following the manufacturer’s instructions (Promega).
Supplementary Material
supplementary figures
Acknowledgements
We thank V. Siegel, K. Mochizuki, G. B. Cebolla and S. Weitzer for comments on the manuscript, J. Stolte for assistance with quantitative PCRs, C. Richter and the other members of the Knoblich laboratory for discussion, B. Dickson, E. Izaurralde, P. Lasko, L. Luo, S. Hawley, D. McKearin, H. Richardson, F. Schnorrer, J. Skeath, D. Stein, L. Wong, the Bloomington Drosophila Stock Center, the Developmental Studies Hybridoma Bank (DSHB) and the Drosophila Genomics Resource Center (DGRC) for reagents, and M. Insco and M. Fuller for communicating results before publication. Work in the Knoblich laboratory is supported by the Austrian Academy of Sciences, the Wiener Wissenschafts-, Forschungs- und Technologiefonds (WWTF), the Austrian Science Fund (FWF) and the EU network ONCASYM; K.M. is supported by the Austrian Proteomics Platform (APP) of the Austrian Genome Program (GENAU).
Appendix
METHODS
Cytology and immunofluorescence
Immunofluorescence experiments in larval brains were carried out as described previously6. Ovaries were dissected in PBS and fixed for 20 min in a 1:1 mixture of _n_-heptane and PBS containing 5% PFA. After three washes in PBS containing 0.1% Triton X-100, they were treated as described for brains6. For in situ hybridizations, DIG-labelled antisense probes were synthesized from the EST GH01646. For clonal analysis, Flp expression was induced by incubating flies at 37 °C for 1 h. hs-bam (Fig. 3d, e) was induced by three consecutive 1 h incubations at 37 °C within one day during development. Overexpression of mei-P26 in the larval brain was carried out with a temperature-sensitive Gal80 (a yeast protein that suppresses Gal4) to circumvent embryonic lethality, and expression was induced for 60 h at 29 °C. The following antibodies were used: mouse anti-1B1 (7H9, Developmental Studies Hybridoma Bank (DSHB), 1:1), rabbit anti-AGO1 (Abcam, 1:100), mouse anti-Armadillo (N2 7A1, DSHB, 1:100), rabbit anti-Brat (1:100), mouse anti-CycE (from H. Richardson, 1:10), guinea pig anti-Deadpan (gift from J. Skeath, unpublished, 1:1,000), mouse anti-Fibrillarin (Abcam, 1:10), mouse anti-GFP (Roche, 1:100), rabbit anti-GFP (Abcam, 1:100), mouse anti-c-Myc (Santa Cruz Biotechnology, 1:100), rabbit anti-d-Myc (from D. Stein 1:5,000), rabbit anti-phosphorylated histone H3 (Upstate Biotechnology, 1:1,000), mouse anti-Prospero (DSHB, 1:10), mouse anti-Orb (4H8, DSHB, 1:10), mouse anti-α-Tubulin (Sigma), rabbit anti-Vasa (gift from P. Lasko, 1:10,000) and goat anti-Vasa (Santa Cruz, 1:200). Rhodamine-conjugated phalloidine (Alexa) was used 1:1,000 and rabbit anti-Mei-P26 (1:300) was raised against the peptide NLKTVLSDDASNSSVLED corresponding to amino acids 23–40. Samples were mounted in Vectashield containing 4,6-diamidino-2-phenylindole (DAPI; Vector Laboratories) and imaged on a Zeiss LSM 500 confocal microscope equipped with a blue diode laser to visualize DAPI. Images were processed in Adobe Photoshop and Adobe Illustrator.
Constructs
The mei-P26 full-length, mei-P26ΔNHL (amino acids 1–933), AGO1, dappled and brat coding sequences were PCR-amplified from total complementary DNA or ESTs with appropriate primers containing attB recombination sites (or with primers containing appropriate restriction sites), and were sequenced and recombined into pDONR221 (Invitrogen) and thereafter recombined into either tagged or untagged pUASp or pUAST destination vectors (DGRC). For fly transformation, constructs were co-injected with Δ2–3 transposase into Drosophila w1118 embryos.
To generate a bantam luciferase reporter, two copies of a sequence complementary to the bantam microRNA were cloned downstream of the FLuc reporter plasmid (bantam sensor). A plasmid expressing Renilla luciferase (RLuc) (gift from E. Izaurralde) served as a transfection control.
Biochemistry and S2 cell experiments
For ovary lysates, 20 ovaries from the respective genotypes were dissected in PBS. Proteins were extracted with Laemmli buffer and run on a 10% SDS-page. S2 cell transfections, immunoprecipitations, silverstaining and mass spectrometry were performed essentially as described6.
For microRNA assays, S2 cells were simultaneously transfected with Actin–Gal4, the FLuc microRNA reporter construct, the RLuc transfection control construct and pUASp mei-P26, pUASp mei-P26ΔNHL or the empty pUASp vector as a control. Dual luciferase assays were performed 24 h after transfection following the manufacturer’s instructions (Promega). For each experiment, transfections were performed in triplicates. Three independent experiments showed comparable results, one of which is shown in Supplementary Fig. 7.
Fly strains
We used the following Drosophila strains: AGO1k08121 (gift from T. Uemura), bantamΔ1, mei-P26fs1 (ref. 8), mei-P26mfs1 (ref. 8), bamΔ86 (Bloomington), loqsf00791 (Bloomington), loqsKO (gift from D. McKearin), nanos-Gal4::VP16 (gift from F. Schnorrer), hs-Bam (gift from D. McKearin), brat192, FRT18A Ubi-GFP.nls, hs-Flp/CyO (Bloomington), yw hsFLP; FRT G13 2xGFP.nls, UASP-mei-P26 (gift from S. Hawley, unpublished), BamP-GFP (gift from D. McKearin), the Gal4 lines OK107 (gift from L. Luo) and 1407, tub-Gal80ts (Bloomington), Rho1–GFP trap (ZCL1957, Fly Trap), and pum01688 (Bloomington), pumET3. All fly strains were raised on standard food without wet or dry yeast.
microRNA quantitative PCRs
Total RNA from ovaries was extracted from the four respective genotypes (wild type, mei-P26mfs1/fs1, bamΔ86 and bamΔ86 nanos-Gal4::VP16 pUASp-mei-P26) using the TRIzol Reagent (Invitrogen). Primer sets designed to amplify mature microRNAs (and sno RNA227 as a control reaction) were obtained from Applied Biosystems. Products were amplified from 10 ng total RNA samples from the respective genotypes with the TaqMan microRNA assay using a quantitative PCR machine (Applied Biosystems). Wild-type microRNA levels are set to zero in Supplementary Fig. 7a.
pUASp-mei-P26) using the TRIzol Reagent (Invitrogen). Primer sets designed to amplify mature microRNAs (and sno RNA227 as a control reaction) were obtained from Applied Biosystems. Products were amplified from 10 ng total RNA samples from the respective genotypes with the TaqMan microRNA assay using a quantitative PCR machine (Applied Biosystems). Wild-type microRNA levels are set to zero in Supplementary Fig. 7a.
Quantification
Wild-type and mei-P26mfs1 flies carrying BamP_–_GFP were stained with mAb1B1 to identify cell types and rhodamine-phalloidine to outline cells. Cell diameters were determined from image stacks of 0.4-μm sections using the measuring tool of LSM software (Zeiss). Cell types were defined as follows: Stem cells: GFP-negative, niche-contacting single-spectrosome-containing cells. Cystoblasts: GFP-positive single-spectrosome-containing cells. Cystocytes: 4, 8 or 16 GFP-positive cells connected by fusomes. To determine nucleolar:cell size ratio, anti-Fibrillarin and rhodamine-phalloidine were used in stainings. For ellipsoid cells, the mean cell diameter was determined (from the longest and shortest cell diameter) and used in the statistics.
For volume reconstruction of germline stem cells, Rho1–GFP trap (ZCL1957) flies were stained with mouse anti-1B1, GFP and DAPI, and volumes were determined from image stacks of 0.4-μm sections using the IMARIS software. For reconstruction of cyst volume, GFP-negative clones, stained with mouse anti–1B1, rhodamine-conjugated phalloidine (which perfectly colocalizes with the Rho1–GFP trap localization pattern within the resolution of our microscope), GFP and DAPI and were analysed in a similar manner.
Footnotes
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
References
- 1.Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583–597. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Fuller MT, Spradling AC. Male and female Drosophila germline stem cells: two versions of immortality. Science. 2007;316:402–404. doi: 10.1126/science.1140861. [DOI] [PubMed] [Google Scholar]
- 3.Gilboa L, Lehmann R. How different is Venus from Mars? The genetics of germ-line stem cells in Drosophila females and males. Development. 2004;131:4895–4905. doi: 10.1242/dev.01373. [DOI] [PubMed] [Google Scholar]
- 4.Lee CY, Wilkinson BD, Siegrist SE, Wharton RP, Doe CQ. Brat is a Miranda cargo protein that promotes neuronal differentiation and inhibits neuroblast self-renewal. Dev. Cell. 2006;10:441–449. doi: 10.1016/j.devcel.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Bello B, Reichert H, Hirth F. The brain tumor gene negatively regulates neural progenitor cell proliferation in the larval central brain of Drosophila. Development. 2006;133:2639–2648. doi: 10.1242/dev.02429. [DOI] [PubMed] [Google Scholar]
- 6.Betschinger J, Mechtler K, Knoblich JA. Asymmetric segregation of the tumor suppressor brat regulates self-renewal in Drosophila neural stem cells. Cell. 2006;124:1241–1253. doi: 10.1016/j.cell.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 7.Sekelsky JJ, et al. Identification of novel Drosophila meiotic genes recovered in a P-element screen. Genetics. 1999;152:529–542. doi: 10.1093/genetics/152.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Page SL, McKim KS, Deneen B, Van Hook TL, Hawley RS. Genetic studies of mei-P26 reveal a link between the processes that control germ cell proliferation in both sexes and those that control meiotic exchange in Drosophila. Genetics. 2000;155:1757–1772. doi: 10.1093/genetics/155.4.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reymond A, et al. The tripartite motif family identifies cell compartments. EMBO J. 2001;20:2140–2151. doi: 10.1093/emboj/20.9.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaccai M, Lipshitz HD. Differential distributions of two adducin-like protein isoforms in the Drosophila ovary and early embryo. Zygote. 1996;4:159–166. doi: 10.1017/s096719940000304x. [DOI] [PubMed] [Google Scholar]
- 11.Lin H, Yue L, Spradling AC. The Drosophila fusome, a germline-specific organelle, contains membrane skeletal proteins and functions in cyst formation. Development. 1994;120:947–956. doi: 10.1242/dev.120.4.947. [DOI] [PubMed] [Google Scholar]
- 12.Ohlmeyer JT, Schupbach T. Encore facilitates SCF-ubiquitin-proteasome-dependent proteolysis during Drosophila oogenesis. Development. 2003;130:6339–6349. doi: 10.1242/dev.00855. [DOI] [PubMed] [Google Scholar]
- 13.Chen D, McKearin DM. A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development. 2003;130:1159–1170. doi: 10.1242/dev.00325. [DOI] [PubMed] [Google Scholar]
- 14.Lantz V, Chang JS, Horabin JI, Bopp D, Schedl P. The Drosophila orb RNA-binding protein is required for the formation of the egg chamber and establishment of polarity. Genes Dev. 1994;8:598–613. doi: 10.1101/gad.8.5.598. [DOI] [PubMed] [Google Scholar]
- 15.Kai T, Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 2004;428:564–569. doi: 10.1038/nature02436. [DOI] [PubMed] [Google Scholar]
- 16.Frank DJ, Edgar BA, Roth MB. The Drosophila melanogaster gene brain tumor negatively regulates cell growth and ribosomal RNA synthesis. Development. 2002;129:399–407. doi: 10.1242/dev.129.2.399. [DOI] [PubMed] [Google Scholar]
- 17.Grewal SS, Li L, Orian A, Eisenman RN, Edgar BA. Myc-dependent regulation of ribosomal RNA synthesis during Drosophila development. Nature Cell Biol. 2005;7:295–302. doi: 10.1038/ncb1223. [DOI] [PubMed] [Google Scholar]
- 18.Rudra D, Warner JR. What better measure than ribosome synthesis? Genes Dev. 2004;18:2431–2436. doi: 10.1101/gad.1256704. [DOI] [PubMed] [Google Scholar]
- 19.Tolia NH, Joshua-Tor L. Slicer and the argonautes. Nature Chem. Biol. 2007;3:36–43. doi: 10.1038/nchembio848. [DOI] [PubMed] [Google Scholar]
- 20.Jin Z, Xie T. Dcr-1 maintains Drosophila ovarian stem cells. Curr. Biol. 2007;17:539–544. doi: 10.1016/j.cub.2007.01.050. [DOI] [PubMed] [Google Scholar]
- 21.Park JK, Liu X, Strauss TJ, McKearin DM, Liu Q. The miRNA pathway intrinsically controls self-renewal of Drosophila germline stem cells. Curr. Biol. 2007;17:533–538. doi: 10.1016/j.cub.2007.01.060. [DOI] [PubMed] [Google Scholar]
- 22.Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113:25–36. doi: 10.1016/s0092-8674(03)00231-9. [DOI] [PubMed] [Google Scholar]
- 23.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 24.Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124:1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Caussinus E, Gonzalez C. Induction of tumor growth by altered stem-cell asymmetric division in Drosophila melanogaster. Nature Genet. 2005;37:1125–1129. doi: 10.1038/ng1632. [DOI] [PubMed] [Google Scholar]
- 26.Lee CY, et al. Drosophila Aurora-A kinase inhibits neuroblast self-renewal by regulating aPKC/Numb cortical polarity and spindle orientation. Genes Dev. 2006;20:3464–3474. doi: 10.1101/gad.1489406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, et al. Aurora-A acts as a tumor suppressor and regulates self-renewal of Drosophila neuroblasts. Genes Dev. 2006;20:3453–3463. doi: 10.1101/gad.1487506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross DT, et al. Systematic variation in gene expression patterns in human cancer cell lines. Nature Genet. 2000;24:227–235. doi: 10.1038/73432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary figures