Reelin, Rap1 and N-cadherin orient the migration of multipolar neurons in the developing neocortex (original) (raw)
. Author manuscript; available in PMC: 2011 Dec 1.
Published in final edited form as: Nat Neurosci. 2011 Apr 24;14(6):697–703. doi: 10.1038/nn.2816
Abstract
Projection neurons migrate from the ventricular zone to the neocortical plate during mouse brain development. Their overall movement is radial, but they become multipolar and move non-radially in the intermediate zone. Here we show that Reelin, the Rap1 GTPase, and N-cadherin (NCad) are important for multipolar neurons to polarize their migration towards the cortical plate. Inhibition and rescue experiments indicate that Reelin regulates migration through Rap1 and Akt, and that Rap1-regulated GTPases, RalA/B, Rac1 and Cdc42, are also involved. We find that Rap1 regulates plasma membrane localization of N-cadherin, and N-cadherin rescues radial polarization when Rap1 is inhibited. Curiously, inhibition of Rap1 or N-cadherin has little effect on glia-dependent locomotion. We propose a multi-step mechanism in which Reelin activates Rap1, Rap1 up-regulates N-cadherin, and N-cadherin is needed to orient cell migration.
The mammalian neocortex is patterned by the coordinated migration of immature neurons 1. The majority of neocortical projection neurons originate by asymmetric division of radial glia progenitors in the ventricular zone (VZ). They then move radially to the sub-ventricular zone and lower intermediate zone (sVZ/IZ), where they become multipolar. They dynamically extend and retract multiple long projections and move in apparently random directions 1–3. Axonogenesis starts as cells approach the middle of the IZ. After the axon emerges, the cells reorient their centrosome and Golgi upwards, towards the developing cortical plate (CP), and resume radial migration 4. As they move to the upper part of the IZ, their morphology changes from multipolar to bipolar. Bipolar cells have a thick, radially-oriented leading process and a thin, trailing axon, and move by locomotion along the radially-oriented processes of radial glia or other neurons 1,5.
The transition from multipolar to radial migration is inhibited by many mutations and experimental manipulations, suggesting that it is a complex process 1. Among the requirements are cytoskeletal regulators including dynein-associated protein and extracellular signals including Semaphorin 3A, but little is known about how the signals are interpreted by the cell 1,6,7.
In many cell types, cell orientation and polarity are controlled by local activation and global inhibition of signaling pathways that organize the cytoskeleton and direct vesicle traffic 8. Positive feedback loops coordinated by small GTPases stabilize cell polarity in response to intracellular and extracellular cues. GTPases are molecular switches that are activated by guanine nucleotide exchange factors (GEFs) and inactivated by GTPase activating proteins (GAPs). The GTP state is active and binds to effector molecules. In yeast and leukocytes, the Ras-related GTPase Rap provides a critical nexus for activating other small GTPases, which in turn regulate the actin cytoskeleton and membrane traffic 8. Rap proteins are also important for polarization of non-motile cells, for example, for specifying axon-dendritic polarity of cultured hippocampal neurons 9 and apical-basolateral polarity of epithelial cells 10. The roles of Rap proteins in vivo in mammals have been unclear, however, perhaps partly because of possible redundancy between the 5 Rap genes (Rap1A, 1B, 2A, 2B and 2C) 11.
Here we have studied the role of Rap proteins in the cortical development, and discovered a key role for Rap1 in polarizing radial migration of multipolar cells. Specifically, we found that Reelin, which is well known for its role in neuron lamination in the cortical plate 1,12, activates Rap1 in multipolar neurons in the intermediate zone. In turn, Rap1 regulates neural cadherin (NCad, or CDH2). NCad is a classical cadherin: a single-pass transmembrane receptor that regulates cell-cell contact by calcium-dependent homophilic binding 13. We find that NCad is needed to orient the migration of multipolar cells towards the cortical plate. Our results suggest a multi-step model for orienting the migration of cortical neurons in the IZ.
Results
Rap regulates migration into the upper intermediate zone
We monitored cortical development in vivo by electroporation of VZ progenitor cells with green fluorescent protein (GFP) 14. We then observed the positions of daughter neurons at various times thereafter. Previous studies have shown that most cells in the lower IZ and sVZ are multipolar, while most cells in the upper IZ and CP are bipolar, so we name these regions the multipolar migration zone (MMZ) and radial migration zone (RMZ), respectively 1–3. Control neurons reach the MMZ a day after electroporation (E15.5) and are still there a day later (E16.5) (Supplementary Fig. S1 on line). During the third day (E17.5), most of the neurons have passed into the RMZ, and some have reached the top of the CP 1–3.
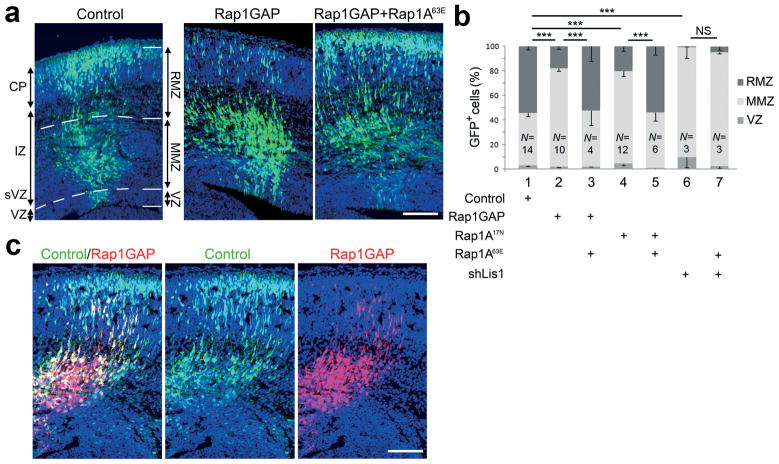
To test the role of Rap proteins in this process, we expressed dominant negative Rap1A17N or Rap1GAP, which inhibits all Rap family members but not Ras 14. Rap-inhibited neurons reached the MMZ normally in the first day, but their movement into the RMZ on the third day was delayed (Fig. 1a and Supplementary Fig. S1 on line). The decreased proportion of cells in the RMZ was highly significant (Fig. 1b). To test whether the defect was due to the inhibition of Rap activity in neurons or in other cells, we expressed Rap1GAP or dominant-negative Rap1A17N from the NeuroD promoter, which is only expressed in post-mitotic neurons (Supplementary Fig. S2 on line). RMZ entry was inhibited (Fig. 1b and Supplementary Fig. S3 on line), indicating that Rap is required in post-mitotic neurons. Moreover, inhibition of RMZ entry by either Rap1GAP or Rap1A17N could be rescued by the co-expression of the active mutant Rap1A63E in post-mitotic neurons (Fig. 1b) while the active form did not affect cell migration when expressed alone (Supplementary Fig. S3 on line). As a further control for specificity, we used shRNA to deplete the dynein regulator Lis1. As reported 7, Lis1 is needed for entry into the RMZ, but this block was not rescued by Rap1A63E (Fig. 1b). Moreover, sequential electroporation of GFP followed by a mixture of cherry fluorescent protein (ChFP) and Rap1GAP showed that Rap-inhibited cells do not affect the migration of control cells (Fig. 1c). To confirm our results, we knocked down Rap1A and Rap1B using specific shRNAs. Knockdown of Rap1A or both Rap1A and Rap1B gave a similar phenotype to that caused by Rap1GAP, while Rap1B had less effect (Supplementary Fig S3a,b on line). The specificity of Rap1A shRNA was confirmed by the rescue of the phenotype when a non targetable Rap1A cDNA was co-expressed (Supplementary Fig S3b on line). Further controls showed that Rap1 inhibition did not detectably affect the Pax6+ apical progenitor cells, Nestin+ radial glia fibers, Tbr2+ basal progenitors in the SVZ, Cux1+ neuronal fate, cell proliferation or apoptosis (Supplementary Fig. S4 on line). These results show that Rap1 family GTPases, principally Rap1A, have a cell-autonomous role in regulating neuron exit from the MMZ.
Figure 1. Rap activity is required cell-autonomously for neuron entry into the upper intermediatezone and cortical plate.
(a–c) Fetal brains were electroporated in utero at embryonic day 14.5 (E14.5) with plasmids expressing the indicated proteins along with GFP or ChFP. Rap1GAP was expressed in progenitors and post-mitotic neurons under the CAG promoter; Rap1A17Nand Rap1A63E were expressed in post-mitotic neurons using the NeuroD promoter. (a) Positions of transfected cells at E17.5. The cerebral wall was subdivided into RMZ (CP and upper IZ), MMZ (middle and lower IZ and sVZ) and VZ (GFP, green; DAPI, blue). (b) Percentage of electroporated cells in each region. (c) Sequential electroporation with GFP, followed, 10 min later, by Rap1GAP and ChFP, shows that Rap role in migration is cell autonomous. Scale bars, 100 μm. RMZ, radial morphology zone; MMZ, multipolar morphology zone; VZ, ventricular zone; CP, cortical plate; IZ, intermediate zone; sVZ, subventricular zone. Error bars, s.e.m. **, P < 0.01; ***, P < 0.001; NS, non significant.
Rap1 orients the migration of multipolar neurons
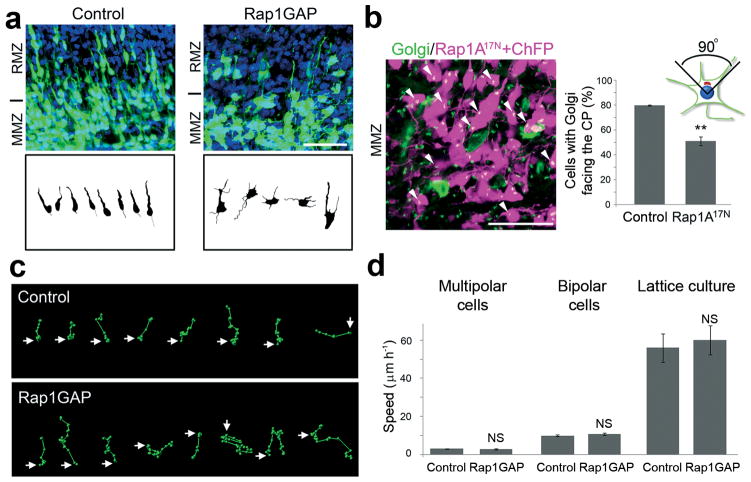
We reasoned that Rap1 might be needed before the start of radial migration. We first checked the morphology of control and Rap-inhibited cells. Two days after electroporation, most control neurons in the lower part of the RMZ, close to the transition zone, had bipolar morphology (90.0 ± 2 %) but most Rap-inhibited neurons were multipolar (10.5 ± 0.5 % with bipolar morphology) (Fig. 2a). We then examined the orientation of the Golgi apparatus in multipolar cells in the upper MMZ by staining for the Golgi protein GM130. In control electroporations, most upper MMZ neurons have their Golgi apparatus oriented towards the CP, but the Golgi was disoriented if Rap was inhibited (Fig. 2b). We then tracked the movement of multipolar neurons in the upper MMZ using time-lapse video-microscopy (Movie 1 in Supplement on line). Most of the multipolar neurons in this region of control cortex moved upwards towards the CP (91.1 ± 1.1 %) (Movie 1 on line), and only a minority moved randomly (typical paths are shown in Fig. 2c). In contrast, most Rap-inhibited neurons moved in random directions (Movie 1 on line) with only 38.7 ± 1.2 % migrating towards the CP. Typical paths are shown in Fig. 2c. Rap inhibition did not affect the speed of multipolar migration (Fig. 2d). These results suggest that Rap activity is needed for multipolar cells to orient their migration towards the CP. Interestingly, Rap-inhibited and Rap1 knockdown neurons still extended axons (Supplementary Fig. S5 on line), suggesting that Rap inhibition does not prevent cortical axonogenesis in vivo. This contrasts with the requirement for Rap1B for hippocampal axonogenesis in vitro 9. However, we cannot rule out that residual Rap activity might be sufficient for axonogenesis in vivo, or there might be a difference in Rap requirement due to the cell type or in vivo versus in vitro system.
Figure 2.
Rap is required to orient multipolar cells but not for migration of bipolar neurons. The indicated plasmids were electroporated in utero at E14.5 along with plasmids expressing GFP or ChFP. (a) Computer-based reconstruction of shapes of GFP-positive neurons in E16.5 cortices at the transition between MMZ and RMZ. (b) Golgi staining (green, arrowheads) of MMZ neurons (magenta). The percentage of cells with Golgi facing the CP was calculated. (c) Tracks of migration paths followed by control and Rap-inhibited multipolar cells in the upper part of the MMZ. Positions of cell centroids in successive frames (circles) are linked by lines. The start position is marked by an arrow. (d) Migration of control and Rap inhibited GFP-positive multipolar (Control: N = 28 cells in 3 movies; Rap1GAP: N = 21 in 3 movies) and bipolar (Control: N = 121 cells in 6 movies; Rap1GAP: N = 51 in 4 movies) neurons in cortical slices prepared at E16.5 from brains electroporated at E14.5. Migration of bipolar GFP-positive cortical neurons in lattice culture (Control: N = 22 cells; Rap1GAP: N = 14). Mean + s.e.m. of average migration speed.. Scale bars, 50 μm. **, P < 0.01; NS, non significant.
The Rap1 requirement for migration was not absolute. Populations of cells expressing Rap1GAP or Rap1A17N were migrating through the CP with bipolar morphology by five days after electroporation (Supplementary Fig. S6a on line). These cells were not normal, since they did not form an apical dendritic tree at the top of the CP (Supplementary Fig. S6b on line). This implies that cells may be capable of locomotion despite low Rap activity. Indeed, time-lapse video-microscopy of the RMZ at E16.5 showed that control and Rap-inhibited cells migrated at similar speed (Fig. 2d and Movie 2 on line). To further test whether Rap is required for neuron migration, we dissociated E15.5 cerebral cortex, electroporated the cells with GFP and Rap1GAP or vector, and measured their migration on lattices comprised of radial glia and neuronal processes, similar to the substrate they encounter in vivo 15. Control and Rap1GAP-expressing cells migrated equally (Fig. 2d and Movie 3 on line). As shown below, Rap1GAP inhibits Rap1 in these cells. Taken together, these results suggest that Rap1 is transiently involved in the orientation of multipolar migration in the IZ, but is unimportant for radial migration through the CP.
Rap regulates N-cadherin localization in cortical neurons
In many cell types, Rap1 regulates traffic between endosomes and the plasma membrane 16. Indeed, epitope-tagged Rap1A was detected on both endosomes and the plasma membrane of electroporated neurons in vivo (Supplementary Fig. S7a on line). Therefore we considered the possibility that Rap1 might regulate cell orientation by controlling the cell-surface expression of membrane proteins that sense the environment.
In other cell types, Rap1 regulates cell-surface adhesion proteins of the integrin and classical cadherin families 11,17,18. The role of integrins in neuronal migration has been studied before and is still unclear 19. Therefore we tested whether cadherins may be involved in neuron migration. We focused on NCad because it is expressed throughout the developing cortex, including the region where radial migration starts (see below).
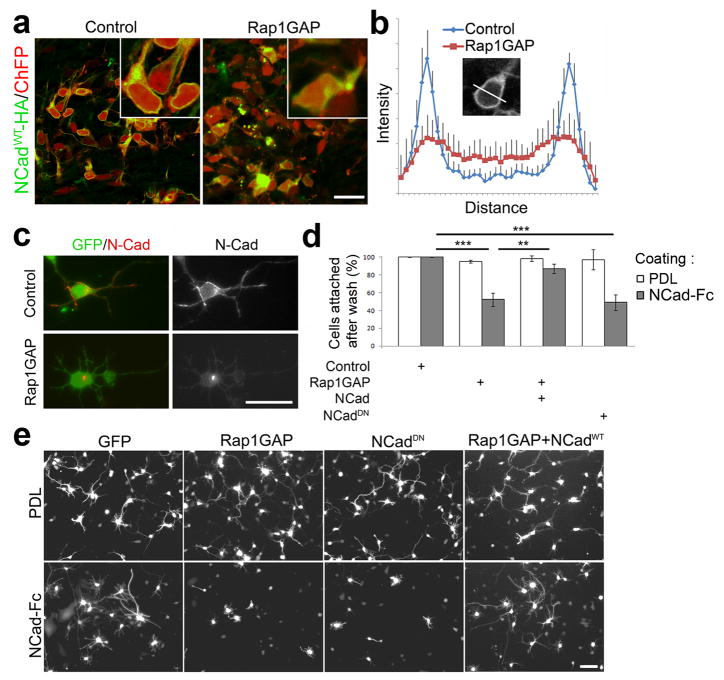
To test whether Rap1 regulates NCad surface levels in vivo we introduced HA-tagged full-length wildtype NCad by in utero electroporation together with control vector or Rap1GAP. NCadWT-HA was detected on the plasma membrane and internal compartments under both conditions, but membrane levels were relatively decreased when Rap1GAP was expressed (Fig. 3a,b). To test whether the localization of endogenous NCad is regulated by Rap, we electroporated embryonic cortical neurons with GFP and Rap1GAP or vector and examined the cells by immunofluorescence. While endogenous NCad localized to the plasma membrane of control cells, it was primarily in a perinuclear structure in Rap1GAP-expressing cells (Fig. 3c). To test whether Rap1 modulates functional NCad exposed on the cell surface, we measured homophilic adhesion of electroporated neurons to surfaces coated with the NCad extracellular domain (NCad-Fc). GFP-positive adherent cells that expressed the neuron marker MAP2 (Supplementary Fig. S8 on line) were counted. While control neurons bound either to NCad-Fc or control poly-D-lysine (PDL), Rap1GAP specifically inhibited neuron binding to NCad-Fc (Fig. 3d). As a control, the active form of Rap1A was able to rescue the binding defect (Supplementary Fig S9a on line). Rap1GAP also inhibited neurite extension on NCad-Fc but not PDL (Fig. 3e). These phenotypes were NCad dependent, since over-expression of full-length, wild-type NCadWT rescued binding and neuritogenesis by Rap1GAP-expressing cells on NCad-Fc (Fig. 3d,e). To inhibit cadherin function we used a mutant NCadDN lacking most of the extracellular domain. Similar mutants have been used in other studies 20,21, and induce internalization of endogenous cadherins 22. As expected, NCadDN inhibited cortical neuron attachment and neuritogenesis on NCad-Fc (Fig. 3d,e). These results suggest that Rap activity maintains functional cadherins on the cell surface of neurons in vivo and in vitro. However these data do not distinguish whether the primary effect is on endocytosis, exocytosis or the overall level of NCad.
Figure 3. Rap regulates adhesion to NCad and membrane NCad levels.
(a) HA antibody staining (green) of MMZ neurons that had been electroporated in utero with HA-tagged NCadWT, ChFP and vector (control) or Rap1GAP. (b) Mean ± s.e.m. of HA-NCad fluorescence intensity profiles across the bodies of N = 9 control and N = 9 Rap1GAP-expressing cells. (c) Immunofluorescence of the endogenous NCad in dissociated cortical neurons electroporated with GFP expressing plasmid alone or along with the Rap1GAP expressing plasmid. (d–e) E15.5 dissociated cortical neurons were electroporated with the indicated plasmids and incubated overnight on dishes coated with NCad-Fc or PDL. (d) GFP-expressing cells were counted before and after washes to determine the percentage of attached cells. (e) The morphology of GFP-expressing attached cells was observed after a further one day of differentiation. Scale bars, 20 μm.
Several Rap effectors regulate cadherins in other systems. The Rap1 effector Vav2 and its substrate GTPase Cdc42 inhibit ECad endocytosis 23, while the Rap1 effector RalGDS activates RalA, which docks secretory vesicles to the exocyst complex and recycles ECad to epithelial cell-cell junctions 24. As a preliminary test of which Rap effectors may regulate NCad in multipolar neurons, we performed in utero electroporation with GTPase binding domains from NWASP, POSH and Sec5, which are semi-specific inhibitors of Cdc42, Rac1 and RalA/B, respectively 25,26. Each of these proteins partially inhibited RMZ entry, suggesting requirements for both Rac1/Cdc42 and RalA/B (Supplementary Fig. S9b on line). To further confirm the involvement of Ral enzymes in cortical development, we knocked down RalA and RalB separately using shRNA. Knock down of either RalA or RalB significantly inhibited RMZ entry. Inhibition was rescued by human RalA or RalB respectively (Supplementary Fig S9b on line). This suggests that both Ral proteins have an important role in this process. Activated Rap1A63E did not overcome inhibition by the POSH binding domain, suggesting that Rap1 is upstream or parallel to Rac1 (Supplementary Fig. S9b on line). We then tested whether activated Vav2, RalA, RalB, Cdc42 and Rac1 could rescue RMZ entry by Rap-inhibited neurons. Vav2 gave almost complete rescue, while each of the small GTPases individually allowed partial rescue without affecting migration when electroporated alone (Supplementary Fig. S9c,d on line). Interestingly, inhibition of Ral enzymes also affected the presence of wildtype NCad-HA at the plasma membrane (Supplementary Fig. S9e on line). Moreover, the active form of Vav2 was able to rescue the NCad-binding defect of Rap inhibited neurons (Supplementary Fig S9a on line). These results suggest that RalA/B and Rac1/Cdc42 regulate NCad traffic and that Rap1 may regulate NCad membrane localization by several mechanisms.
Cadherins regulate radial orientation of multipolar neurons
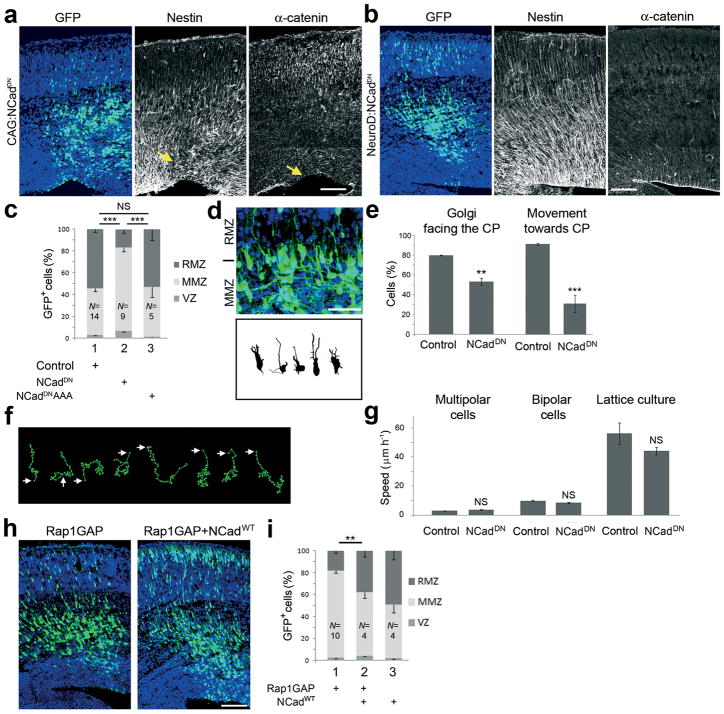
We next tested whether cadherins regulate multipolar neuron migration by in utero electroporation of NCadDN. Expression of NCadDN in progenitor cells using the ubiquitous CAG promoter disrupted α-catenin+ apical junctions and Nestin+ radial glia (Fig. 4a), as expected 27. However, expression of NCadDN in post-mitotic neurons from the NeuroD promoter inhibited multipolar neurons in the MMZ (Fig. 4b,c), without affecting apical junctions or radial glia (Fig. 4b). A mutant NCadDN, NCadDNAAA, which binds α and β but not p120Ctn 28, did not inhibit neuron movement, consistent with p120Ctn regulating cadherin traffic 22, and suggesting that αand β-catenin are not needed, or are not limiting, for NCad function (Fig. 4c).
Figure 4. NCad is required to orient multipolar cells, under control of Rap1.
(a–b) Electroporation of a dominant-negative NCadDN in progenitor cells (a) or post-mitotic cells (b) at E14.5 and labeled for the indicated proteins 3 days later. (c) Percentage of electroporated cells in each region (mean + s.e.m). NCadDNAAA is an inactive point mutant of NCadDN. (d) Computer-based reconstruction of shapes of GFP-positive neurons in E16.5 cortices at the transition between MMZ and RMZ. (e) Percentage of cells with Golgi facing the CP and fraction of multipolar neurons migrating towards the CP in time-lapse videomicroscopy. (f) Tracks of migrating NCadDN-expressing multipolar cells in the upper part of the MMZ. Positions of cell centroids in successive frames (circles) are linked by lines. The start position is marked by an arrow. (g) Migration of GFP-positive neurons in cortical slices prepared at E16.5 from brains electroporated at E14.5. Cadherin-inhibited multipolar (Control: N = 28 cells in 3 movies; NCadDN: N = 27 in 3 movies) and bipolar (Control: N = 121 cells in 6 movies; NCadDN: N = 65 cells in 4 movies) neurons migrate at a normal speed. Migration of GFP-positive neurons in lattice culture (N =16 cells). Mean + s.e.m. of average migration speed. (h) Wildtype NCadWT, expressed from the NeuroD promoter, partly rescues the migration defect of Rap1GAP-expressing cells. (i) Percentage of electroporated cells in each region (mean + s.e.m.). Scale bars represent 20 μm (d) and 100 μm (a, b, h). **, P < 0.01; ***, P < 0.001; NS, non significant.
More detailed study revealed further similarities between Rap-inhibited cells and NCad-inhibited cells. NCadDN-expressing cells in the upper transition zone between MMZ and RMZ retained multipolar morphology (only 21.1 ± 1.1 % exhibited a bipolar morphology) (Fig. 4d), Golgi orientation was impaired (Fig. 4e), and multipolar neuron migration was disoriented but at a normal speed (Fig. 4e–g). Moreover, like Rap inhibitors, NCadDN did not affect the speed of migration of bipolar neurons in the CP or of bipolar neurons migrating in lattice culture (Fig. 4g).
Importantly, full length NCadWT partly rescued the migration of Rap-inhibited neurons (Fig. 4h,i). Taken together, these results suggest that Rap and NCad are on the same signaling pathway, with Rap regulating cell surface localization of NCad. Immunofluorescence microscopy showed that, like HA-Rap1A63E (Supplementary Fig. S7a on line), NCadWT-HA is present on the surface and in a perinuclear compartment (Supplementary Fig. S7b on line). We therefore suggest that Rap might regulate the presence of NCad to the cell surface, and NCad might be responsible for orienting the migration of multipolar cells out of the MMZ.
Reelin receptors regulate Rap and N-cadherin i
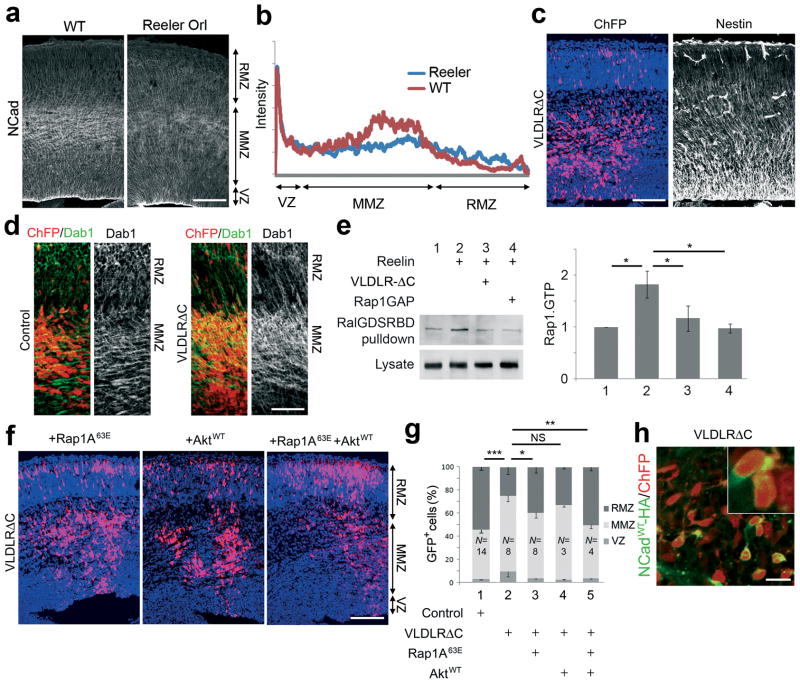
Previous studies have shown that the secreted signaling protein, Reelin, stimulates Rap1 in cultured neurons 29. Reelin is secreted by Cajal-Retzius neurons above the top of the CP, but Reelin cleavage products diffuse to the bottom of the CP 30. Furthermore, some evidence suggests that Reelin affects neurons in the IZ. First, migrating neurons down-regulate their Reelin receptors when they reach the upper IZ 31. Second, mutations in the Reelin-response pathway inhibit exit from the IZ 32,33. Moreover, we found that NCad protein levels are lower in the MMZ of Reelin mutant than wild-type (Fig 5a,b). We therefore tested whether Reelin might be required for Rap activation at the time when multipolar neurons commence radial migration.
Figure 5. Reelin regulates Rap and entry into the radial migration zone.
(a) Immunostaining for NCad in the cerebral wall of wildtype and Reeler mutant embryos at E16.5. (b) Quantification of the NCad staining for 2 brains in each genotype. (c) Expression of dominant-negative VLDLRΔC interferes with neuron entry to the RMZ without affecting radial glia morphology. Left: red, ChFP; blue: DAPI. Right: Nestin. (d) Dominant-negative VLDLRΔC interferes with Reelin-dependent Dab1 degradation in the MMZ. In utero electroporation with ChFP and vector or VLDLRΔC plasmids, stained for ChFP (red) and Dab1 protein (green).(e) Rap1.GTP loading. Neurons were electroporated with HA-Rap1AWT and GFP-RalGDSRBD along with Rap1GAP or VLDLRΔC. Five days after electroporation, neurons were treated with control (lane 1) or Reelin containing-supernatants (lanes 2–4) for 20 min prior to lysis. Lysates were analyzed directly or after pull-down with GFP-RalGDS-RBD to purify Rap1.GTP. Samples were immunoblotted with HA antibodies. The graph shows the Rap.GTP levels from 2 experiments. Similar results were obtained in two additional experiments with stimulation 3 days after electroporation. (f) Rap1 and Akt partly rescue inhibition by VLDLRΔC. (g) Percentage of electroporated cells in each region (mean + s.e.m.). (h) HA antibody staining (green) of MMZ neurons that had been electroporated in utero with HA-tagged NCad, ChFP and VLDLRΔC. Scale bars, 100 μm (a, c, f), 50 μm (d) and 20 μm (h). *, P < 0.05, **, P < 0.01; ***, P < 0.001; NS, non significant.
We prepared a dominant-negative mutant of one of the Reelin receptors, very low density lipoprotein receptor (VLDLR), by deleting part of its cytoplasmic domain. We reasoned that this construct, VLDLRΔC, would compete with endogenous Reelin receptors for Reelin but would not relay the intracellular signal. When VLDLRΔC was expressed from the CAG promoter by in utero electroporation, there was no detectable effect on Nestin+ radial glia, but neurons expressing VLDLRΔC were delayed in the MMZ (Fig. 5c,g). We tested whether VLDLRΔC was inhibiting the Reelin response by assaying the level of the intracellular Reelin signal mediator, Dab1. Reelin stimulates the degradation of Dab1 by the ubiquitin-proteasome system, and Dab1 levels are decreased when Reelin is present 34,35. Immunofluorescent staining showed that multipolar neurons expressing VLDLRΔC contained more Dab1 protein than corresponding control neurons, consistent with a reduced Reelin response in vivo (Fig. 5d). We also tested whether VLDLRΔC inhibits Reelin-induced Rap1 activation by using a pull-down assay in dissociated neurons. VLDLRΔC inhibited Reelin-stimulated Rap1 activation, similarly to Rap1GAP (Fig. 5e).
To test the relationship between Reelin, Rap1 and NCad in MMZ neurons, we first tested whether we could rescue MMZ neurons with blocked Reelin receptors by expressing active Rap1A63E. Indeed, Rap1A63E partly rescued radial migration by Reelin-inhibited cells (Fig. 5f,g). However, almost complete rescue was achieved by co-expressing Rap1A63E with the protein kinase Akt (Fig. 5f,g), which is also involved in Reelin signaling 36,37. Akt alone had little effect when used at this level (Fig. 5f,g) even though over-expression of AktWT is able to increase Akt signaling (Supplementary Fig. S10 on line). These results suggest that Reelin is important to regulate Rap and Akt in MMZ neurons. Second, we tested whether Reelin is involved in up-regulation of NCad on the cell surface. Indeed, membrane levels of NCadWT-HA were decreased in VLDLRΔC-expressing cells in the multipolar zone (Fig. 5h). Reeler mutant neurons were previously found to form distinctive aggregates in culture 38, and changes in neuron-neuron interactions were theorized to underlie the altered development of the Reeler mutant neocortex 39. Our results suggest that altered Rap and NCad levels may underlie these prior studies of Reeler mutant cortex.
Discussion
Our results are consistent with a multi-step model that regulates the radial orientation of multipolar neurons. First, randomly moving multipolar neurons near the middle of the IZ encounter Reelin, and perhaps other signals, that activate Rap1 GTPases (Supplementary Fig. S11 on line, step 1). The active Rap1 then increases the level of NCad on the surface (step 2). It is not clear exactly how Rap1 regulates NCad, but the cytoskeleton and vesicle traffic may be involved because Rho- and Ral-family GTPases play a role. We suspect that Rap1 may regulate NCad endocytosis, exocytosis or stability on the membrane, although other mechanisms cannot be excluded. Surface NCad then allows cells to sense environmental signals that orient the neuron towards the CP, and the multipolar cells migrate radially into the upper IZ (step 3). The nature of the signaling mediated by cell surface NCad is unclear at this time. Finally, after the cells change to bipolar morphology and begin locomotion along radial glia, they become relatively independent of Rap and NCad for movement. Other molecules, such as Connexin-43, may take over to provide adhesive forces for glial-guided locomotion 40.
When our work was nearly complete, a study implicated NCad and Rab-regulated vesicle trafficking in neuronal migration 41. Like us, the authors found that NCad is required for neuron migration, but did not test whether NCad could overcome a block to migration caused by inhibiting recycling. Instead, they found that partial knockdown of NCad would rescue neurons in which NCad endocytosis was blocked by knockdown of Rab5. This suggests that excess surface NCad inhibits migration. We agree, in that high level over-expression of NCad blocks neurons in the multipolar zone. However, we also found that expression of NCad at lower levels, too low to cause a phenotype on its own, is able to rescue Rap1-inhibited neurons. Our results suggest a chain of causality, with Rap1 increasing surface NCad levels on multipolar cells so that the cells can sense directional signals in the environment. We also went further by providing an extracellular signal that regulates NCad surface localization, namely, Reelin.
Our analysis made use of Rap1GAP, NCadDN and VLDLRΔC to interfere with Rap1, NCad and Reelin receptors, respectively. This allowed us to manipulate signaling in post-mitotic neurons specifically and to regulate groups of paralogs simultaneously, but raises concerns about specificity. The following results support the specificity of the reagents. First, for Rap1, we observed the same phenotypes whether we inhibited Rap1 with Rap1GAP or Rap117N, or knocked down Rap1A and Rap1B with shRNA. Moreover, Rap1A63E rescued the migration block caused by Rap1GAP, Rap117N or Reelin inhibition but not the block by Lis1 shRNA or Rac1 inhibition. Next, for NCad, another group found that NCad shRNA caused a migration arrest in the IZ 41, resembling the arrest we detected using NCadDN. Moreover, wildtype NCad rescued neurons in which Rap1 signaling was inhibited. Finally, for Reelin signaling, recent studies support our finding that neurons are delayed in the IZ when Reelin signaling is absent 32,33.
The proposed role for NCad in orienting multipolar cells is reminiscent of the role of ECad in setting up apical-basolateral polarity during the mesenchymal-epithelial transition 10. When migrating mesenchymal cells first touch, ECad forms trans-dimers and generates signals that recruit the actin cytoskeleton and focus active ECad into the junctional region 10. Trans-interacting ECad, or other cell surface receptors such as nectins, locally activate Rap1, and create a positive feedback loop that routes more ECad to the junctions, stabilizes the junctions, and interacts with other receptors and signaling molecules that maintain cell polarity. By analogy, we speculate that NCad on multipolar cells may sense directional cues in the environment by forming trans-dimers with NCad or other cadherins expressed on radially-oriented processes from other neurons or radial glia. However, it is also possible that NCad may interact with other cell surface receptors that respond to directional signals from the cortical plate.
A positive role for NCad in migration is somewhat surprising. In general, cadherins are down-regulated before cell migration 13,42. Indeed, NCad is down-regulated when neurons leave the VZ 43. However, there are a few examples where cadherins are required for cell migration. In some cases, cadherins may provide traction forces between moving and stationary cells, and in other cases they connect groups of cells undergoing collective migration 20,21,44. During the group migration of Drosophila border cells, DECad is required on the border cells as well as the cells they migrate through, and DECad is needed for the first cell of the group to extend a leading process along a growth factor gradient 45. Exactly how this happens is unclear. However, it is possible that surface NCad on multipolar neurons allows them to sense a chemoattractant. It will be interesting to dissect downstream signaling mechanisms from NCad that orient multipolar cell migration in the mammalian neocortex.
While this paper was under review, Reelin, Rap1 and cadherins were reported to regulate somal translocation of early-born cortical neurons 46. Apparently, cadherins stabilize the leading process in the marginal zone. Taken together with our results, it is likely that Reelin, Rap1 and cadherins mediate two key events during cortical development: glia-independent somal translocation of early-born neurons and radial migration of multipolar late-born neurons.
Online Methods
Mice
Experiments were approved by the Hutchinson Center Institutional Animal Care and Use Committee and performed using CD1 mice from Charles-River Laboratories or Reeler (_Reln_rl-Orl) mice that had been backcrossed into the Balb/c genetic background.
Vector construction
Plasmids containing the coding sequences for Rap1GAP, Rap1A17N, Rap1A63E, VAV2DPC, Rac161L, Cdc4261L, NWASP-RBD-GFP, POSH-RBD-GFP (W.T. Arthur); RalA72L, RalB72L (Addgene); Sec5-RBD-GFP (M. White); NCad (V. Vasioukhin) were used as templates to insert the sequences into pCAGIG (K. Sanada) or pNeuroD (F. Polleux). shRNA for Lis1 (L.-H. Tsai) was expressed from the H1 promoter in pCAGGFP. shRNA sequences targeting Rap1A 47 and Rap1B 9 sequences were introduced into the pSUPERretro vector (OligoEngine). The RNAi construct for Rap1B pSHAG-Rap1B was provided by A Puschel 9. RalA and RalB shRNA expression vectors were purchased from Open Biosystems. pCAGGFP (Addgene) and pCAGCherryFP (J. Cooper) were used for co-electroporation. NCadDN contains the transmembrane and intracellular domains (deletion of residues 99-708). A soluble form containing the intracellular domain alone gave similar results (residues 747-906). The AAA mutation was inserted by PCR replacing the GGTGGAGGA sequence at residues 777-779 with GCGGCCGCA. HA tags were at the C-terminus of NCad and the N-terminus of Rap1A and Rap1B. pCAG-Akt1 wildtype was provided by Y. Gotoh (University of Tokyo). Mouse VLDLRΔC, lacking part of the cytoplasmic domain including the Dab1 binding site by deletion of residues 828-873, was cloned by PCR into pCAG-IRES-ChFP (J. Cooper).
Antibodies
For immunofluorescence, we used rabbit anti-Cux1 (Santa Cruz Biotechnology), rabbit anti-Tbr2 (Robert Hevner, Seattle Children’s Hospital Research Institute, Seattle, WA), mouse anti-Nestin (Millipore), cleaved caspase-3 (Cell Signaling), Pax6 (Developmental Studies Hybridoma Bank), mouse anti-HA (Covance), Ki67 (Novacastra), GM130 (BD Biosciences), αN-catenin (Developmental Studies Hybridoma Bank), rabbit anti-MAP2 (Chemicon), mouse anti NCad (Invitrogen) and donkey secondary antibodies labeled with Alexa 488, 568, and 647 (Invitrogen).
In utero microinjection and electroporation
In utero microinjection and electroporation was performed at E14.5 essentially as described previously 48, using timed pregnant CD-1 mice (Charles River Laboratories). Needles for injection were pulled from Wiretrol II glass capillaries (Drummond Scientific) and calibrated for 1 μl injections. DNA solutions were mixed in 10 mM Tris, pH 8.0, with 0.01% Fast Green. Forceps-type electrodes (Nepagene) with 5 mm pads were used for electroporation (five 50 ms pulses of 25 V at E12.5 or five 50 ms pulses of 40 V at E14.5).
Histology and cytology
Embryos were collected at E17.5 or E19.5. Brains were dissected and successful electroporations identified by epifluorescence microscopy. Positive brains were fixed in a 3.7% paraformaldehyde (PFA) in PBS and cryoprotected in a 30% sucrose PBS solution overnight at 4°C. Brains were frozen in optimal cutting temperature compound (OCT) before 14-μm-thick brain cross-sections were obtained with a cryostat and placed on slides. For selected antibodies, sections were antigen retrieved by immersion of the slides in 0.01 M sodium citrate buffer, pH 6.0, at 95°C for 20 min. Dissociated neurons were fixed for 20 min in 3.7% PFA PBS solution. Sections and fixed neurons were permeabilized for 30 min in PBS 0.4% Triton X-100 then blocked for 2 h with 5% normal goat serum and 0.4% Triton X-100 in PBS at room temperature. Primary antibodies were incubated overnight at 4°C. Slides were washed four times for 10 min with 0.4% Triton X-100 in PBS. Secondary antibodies were added for 2 h at room temperature. Slides were washed as before and coverslipped with 0.1 M Tris pH 8.5, 25% glycerol, 10% Mowiol 4-88 (Calbiochem) including 0.1% Diazobicyclo-octane (Sigma) as anti-fade reagent. Most images were obtained with a ×20 objective and were captured using Zeiss LSM 510META confocal microscope with Zeiss LSM Image Browser. Images were assembled in Adobe Photoshop. Brightness and contrast were adjusted equally on figures.
Organotypic slice culture and time-lapse confocal microscopy
Embryonic brains were electroporated at E14.5, and 300 μm embryonic brain slices were prepared at E16.5 using a Vibratome (World Precision Instruments), as described previously 3,49. Time-lapse confocal microscopy was performed using an Achroplan ×20/0.50 with a Zeiss LSM 5 Pascal confocal on an Axioskop2 upright microscope. Slices were embedded in a drop of 3% agarose and cultured in a chamber on a heated stage (Warner Instruments) in DMEM-F12 (Invitrogen) supplemented with B27 (Invitrogen) and 10% serum. The medium was preheated at 37°C and equilibrated with 95% O2 and 5% CO2. The medium was flowed in the chamber at ~5 ml/h. Repetitive acquisitions were performed every 30 min for up to 20 h in latero-dorsal regions of the cortex in which 25 successive “z” optical planes spanning 120 μm were acquired. Z-stacks were selected and combined in Zeiss LSM Image Browser. Slight drifts of the slices were corrected using the ImageJ registration tool Turboreg (Philippe Thévenaz, Biomedical Imaging Group, Swiss Federal Institute of Technology, Lausanne, Switzerland). Average velocity of migrating cells was obtained using Imaris (Bitplane). Histograms were compiled using Microsoft Excel.
Lattice culture
Dorsal neocortices were dissected and cortical cultures were prepared at embryonic day 14.5 (E14.5) using Accutase (Sigma) dissociation. After electroporation (Amaxa) cells were plated in DMEM-F12 medium with 2% B27, 1× Glutamax, 1× Penicillin-Streptomycin, and 30 mM glucose (all reagents from Invitrogen) on 96-well plates coated with 25 μg/ml poly-D-lysine (Sigma). Cultures were maintained in a 37°C, 5% CO2 incubator. After 1 day in vitro (DIV), lattice cultures were induced with Invitrogen 293 SFM (20% v/v) as described 15. Movies were acquired on a Cellomics Arrayscan with 5 min time interval between frames.
Cortical neuron culture, in vitro electroporation and binding assay
Binding of neurons to N-cadherin extracellular domain-Fc (NCadECD-Fc) fusion protein (human) (R&D Systems #1388-NC) was done by a procedure adapted from 50. N-CadECD-Fc was diluted in HBSS+1 mM CaCl2 and aliquots of 50 μg/ml were stored at −80°C in tubes blocked with 3% BSA. 96-well ELISA high binding plates (Corning #9018) were coated with 12.5 μg/ml NCadECD-Fc or 20 μg/ml poly-D-lysine, overnight at 4°C. Wells were then blocked with 3% BSA for 2h at RT or at 4°C, then washed 3 times with HBSS + 1.2 mM CaCl2. Neuron suspensions were prepared from embryonic day 15.5 (E15.5) mouse embryo telencephalons and electroporated using Amaxa electroporator as described by the manufacturer. Equal cell numbers (105 cells) from each electroporation were plated in each well of the prepared plates. Cells were cultured overnight in DMEM-F12 medium with 2% B27 and 1× Penicillin-Streptomycin, counted, vigorously washed then counted again. Cells remaining on the plate were incubated for 1 more day to assess neurite outgrowth.
Statistical analysis
Statistical analysis made use of Student's t test across N samples, where N is the number of embryos or cells as defined in the figure legends.
Supplementary Material
1
Acknowledgments
We gratefully acknowledge L. Buck for the use of her confocal microscope; W. Arthur, R. Hevner, F. Polleux, K. Sanada, L.-H. Tsai, V. Vasioukhin, A. Puschel, Y. Gotoh, P. Phelps and M. White for reagents; J. Wang and E. Jhingan for technical assistance; A. Goffinet and F. Polleux for helpful discussions; V. Vasioukhin, S. Parkhurst, B. Edgar and S. Simo for helpful comments on the manuscript. This work was supported by the Fonds National de la Recherche Scientifique, Belgium (Y.J.), the European Commission under the Marie Curie International Outgoing Fellowship Program through the Université Catholique de Louvain (Y.J.) and NIH grant CA41072 (J.A.C.).
Footnotes
Author contributions
YJ performed the experiments. YJ and JAC conceived the experiments and wrote the paper.
The authors declare no competing financial interests.
References
- 1.Bielas S, Higginbotham H, Koizumi H, Tanaka T, Gleeson JG. Cortical neuronal migration mutants suggest separate but intersecting pathways. Annu Rev Cell Dev Biol. 2004;20:593–618. doi: 10.1146/annurev.cellbio.20.082503.103047. [DOI] [PubMed] [Google Scholar]
- 2.Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–44. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- 3.Tabata H, Nakajima K. Multipolar migration: the third mode of radial neuronal migration in the developing cerebral cortex. J Neurosci. 2003;23:9996–10001. doi: 10.1523/JNEUROSCI.23-31-09996.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Anda FC, Meletis K, Ge X, Rei D, Tsai LH. Centrosome motility is essential for initial axon formation in the neocortex. J Neurosci. 30:10391–406. doi: 10.1523/JNEUROSCI.0381-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nadarajah B, Brunstrom JE, Grutzendler J, Wong RO, Pearlman AL. Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci. 2001;4:143–50. doi: 10.1038/83967. [DOI] [PubMed] [Google Scholar]
- 6.Chen G, et al. Semaphorin-3A guides radial migration of cortical neurons during development. Nat Neurosci. 2008;11:36–44. doi: 10.1038/nn2018. [DOI] [PubMed] [Google Scholar]
- 7.Tsai JW, Chen Y, Kriegstein AR, Vallee RB. LIS1 RNA interference blocks neural stem cell division, morphogenesis, and motility at multiple stages. J Cell Biol. 2005;170:935–45. doi: 10.1083/jcb.200505166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park HO, Bi E. Central roles of small GTPases in the development of cell polarity in yeast and beyond. Microbiol Mol Biol Rev. 2007;71:48–96. doi: 10.1128/MMBR.00028-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwamborn JC, Puschel AW. The sequential activity of the GTPases Rap1B and Cdc42 determines neuronal polarity. Nat Neurosci. 2004;7:923–9. doi: 10.1038/nn1295. [DOI] [PubMed] [Google Scholar]
- 10.Nelson WJ. Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem Soc Trans. 2008;36:149–55. doi: 10.1042/BST0360149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kooistra MR, Dube N, Bos JL. Rap1: a key regulator in cell-cell junction formation. J Cell Sci. 2007;120:17–22. doi: 10.1242/jcs.03306. [DOI] [PubMed] [Google Scholar]
- 12.Jossin Y. Neuronal migration and the role of reelin during early development of the cerebral cortex. Mol Neurobiol. 2004;30:225–51. doi: 10.1385/MN:30:3:225. [DOI] [PubMed] [Google Scholar]
- 13.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–57. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 14.Rubinfeld B, et al. Molecular cloning of a GTPase activating protein specific for the Krev-1 protein p21rap1. Cell. 1991;65:1033–42. doi: 10.1016/0092-8674(91)90555-d. [DOI] [PubMed] [Google Scholar]
- 15.Nichols AJ, Carney LH, Olson EC. Comparison of slow and fast neocortical neuron migration using a new in vitro model. BMC Neurosci. 2008;9:50. doi: 10.1186/1471-2202-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bivona TG, et al. Rap1 up-regulation and activation on plasma membrane regulates T cell adhesion. J Cell Biol. 2004;164:461–70. doi: 10.1083/jcb.200311093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boettner B, Van Aelst L. Control of cell adhesion dynamics by Rap1 signaling. Curr Opin Cell Biol. 2009;21:684–93. doi: 10.1016/j.ceb.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takai Y, Nakanishi H. Nectin and afadin: novel organizers of intercellular junctions. J Cell Sci. 2003;116:17–27. doi: 10.1242/jcs.00167. [DOI] [PubMed] [Google Scholar]
- 19.Belvindrah R, Graus-Porta D, Goebbels S, Nave KA, Muller U. Beta1 integrins in radial glia but not in migrating neurons are essential for the formation of cell layers in the cerebral cortex. J Neurosci. 2007;27:13854–65. doi: 10.1523/JNEUROSCI.4494-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rieger S, Senghaas N, Walch A, Koster RW. Cadherin-2 controls directional chain migration of cerebellar granule neurons. PLoS Biol. 2009;7:e1000240. doi: 10.1371/journal.pbio.1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taniguchi H, Kawauchi D, Nishida K, Murakami F. Classic cadherins regulate tangential migration of precerebellar neurons in the caudal hindbrain. Development. 2006;133:1923–31. doi: 10.1242/dev.02354. [DOI] [PubMed] [Google Scholar]
- 22.Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J Cell Biol. 2003;163:525–34. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuroda S, et al. Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin- mediated cell-cell adhesion. Science. 1998;281:832–5. doi: 10.1126/science.281.5378.832. [DOI] [PubMed] [Google Scholar]
- 24.Grindstaff KK, et al. Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell. 1998;93:731–40. doi: 10.1016/s0092-8674(00)81435-x. [DOI] [PubMed] [Google Scholar]
- 25.Arthur WT, Quilliam LA, Cooper JA. Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors. J Cell Biol. 2004;167:111–22. doi: 10.1083/jcb.200404068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moskalenko S, et al. The exocyst is a Ral effector complex. Nat Cell Biol. 2002;4:66–72. doi: 10.1038/ncb728. [DOI] [PubMed] [Google Scholar]
- 27.Kadowaki M, et al. N-cadherin mediates cortical organization in the mouse brain. Dev Biol. 2007;304:22–33. doi: 10.1016/j.ydbio.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Kojima S, Borisy GG, Green KJ. p120 catenin associates with kinesin and facilitates the transport of cadherin-catenin complexes to intercellular junctions. J Cell Biol. 2003;163:547–57. doi: 10.1083/jcb.200305137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ballif BA, et al. Activation of a Dab1/CrkL/C3G/Rap1 pathway in Reelin-stimulated neurons. Curr Biol. 2004;14:606–10. doi: 10.1016/j.cub.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 30.Jossin Y, Gui L, Goffinet AM. Processing of Reelin by embryonic neurons is important for function in tissue but not in dissociated cultured neurons. J Neurosci. 2007;27:4243–52. doi: 10.1523/JNEUROSCI.0023-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uchida T, et al. Downregulation of functional Reelin receptors in projection neurons implies that primary Reelin action occurs at early/premigratory stages. J Neurosci. 2009;29:10653–62. doi: 10.1523/JNEUROSCI.0345-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morimura T, Ogawa M. Relative importance of the tyrosine phosphorylation sites of Disabled-1 to the transmission of Reelin signaling. Brain Res. 2009;1304:26–37. doi: 10.1016/j.brainres.2009.09.087. [DOI] [PubMed] [Google Scholar]
- 33.Simo S, Jossin Y, Cooper JA. Cullin 5 regulates cortical layering by modulating the speed and duration of Dab1-dependent neuronal migration. J Neurosci. 2010;30:5668–76. doi: 10.1523/JNEUROSCI.0035-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rice DS, et al. Disabled-1 acts downstream of Reelin in a signaling pathway that controls laminar organization in the mammalian brain. Development. 1998;125:3719–29. doi: 10.1242/dev.125.18.3719. [DOI] [PubMed] [Google Scholar]
- 35.Arnaud L, Ballif BA, Cooper JA. Regulation of protein tyrosine kinase signaling by substrate degradation during brain development. Mol Cell Biol. 2003;23:9293–302. doi: 10.1128/MCB.23.24.9293-9302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beffert U, et al. Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3beta. J Biol Chem. 2002;277:49958–64. doi: 10.1074/jbc.M209205200. [DOI] [PubMed] [Google Scholar]
- 37.Jossin Y, Goffinet AM. Reelin signals through phosphatidylinositol 3-kinase and Akt to control cortical development and through mTor to regulate dendritic growth. Mol Cell Biol. 2007;27:7113–24. doi: 10.1128/MCB.00928-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogawa M, et al. The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron. 1995;14:899–912. doi: 10.1016/0896-6273(95)90329-1. [DOI] [PubMed] [Google Scholar]
- 39.Goffinet AM. An early development defect in the cerebral cortex of the reeler mouse. A morphological study leading to a hypothesis concerning the action of the mutant gene. Anat Embryol (Berl) 1979;157:205–16. doi: 10.1007/BF00305160. [DOI] [PubMed] [Google Scholar]
- 40.Elias LA, Wang DD, Kriegstein AR. Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007;448:901–7. doi: 10.1038/nature06063. [DOI] [PubMed] [Google Scholar]
- 41.Kawauchi T, et al. Rab GTPases-dependent endocytic pathways regulate neuronal migration and maturation through N-cadherin trafficking. Neuron. 2010;67:588–602. doi: 10.1016/j.neuron.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–5. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, et al. Cortical neural precursors inhibit their own differentiation via N-cadherin maintenance of beta-catenin signaling. Dev Cell. 2010;18:472–479. doi: 10.1016/j.devcel.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang X, Chrisman H, Weijer CJ. PDGF signalling controls the migration of mesoderm cells during chick gastrulation by regulating N-cadherin expression. Development. 2008;135:3521–30. doi: 10.1242/dev.023416. [DOI] [PubMed] [Google Scholar]
- 45.Fulga TA, Rorth P. Invasive cell migration is initiated by guided growth of long cellular extensions. Nat Cell Biol. 2002;4:715–9. doi: 10.1038/ncb848. [DOI] [PubMed] [Google Scholar]
- 46.Franco SJ, Martinez-Garay I, Gil-Sanz C, Harkins-Perry SR, Muller U. Reelin regulates cadherin function via Dab1/Rap1 to control neuronal migration and lamination in the neocortex. Neuron. 2011;69:482–97. doi: 10.1016/j.neuron.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu C, et al. Ras is required for the cyclic AMP-dependent activation of Rap1 via Epac2. Mol Cell Biol. 2008;28:7109–25. doi: 10.1128/MCB.01060-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tabata H, Nakajima K. Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neuroscience. 2001;103:865–72. doi: 10.1016/s0306-4522(01)00016-1. [DOI] [PubMed] [Google Scholar]
- 49.Jossin Y, Ogawa M, Metin C, Tissir F, Goffinet AM. Inhibition of SRC family kinases and non-classical protein kinases C induce a reeler-like malformation of cortical plate development. J Neurosci. 2003;23:9953–9. doi: 10.1523/JNEUROSCI.23-30-09953.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qin Y, Capaldo C, Gumbiner BM, Macara IG. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J Cell Biol. 2005;171:1061–71. doi: 10.1083/jcb.200506094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1