Omega-3 fatty acids lower blood pressure by directly activating large-conductance Ca2+-dependent K+ channels (original) (raw)
Abstract
Long-chain polyunsaturated omega-3 fatty acids such as docosahexaenoic acid (DHA), found abundantly in oily fish, may have diverse health-promoting effects, potentially protecting the immune, nervous, and cardiovascular systems. However, the mechanisms underlying the purported health-promoting effects of DHA remain largely unclear, in part because molecular signaling pathways and effectors of DHA are only beginning to be revealed. In vascular smooth muscle cells, large-conductance Ca2+- and voltage-activated K+ (BK) channels provide a critical vasodilatory influence. We report here that DHA with an EC50 of ∼500 nM rapidly and reversibly activates BK channels composed of the pore-forming Slo1 subunit and the auxiliary subunit β1, increasing currents by up to ∼20-fold. The DHA action is observed in cell-free patches and does not require voltage-sensor activation or Ca2+ binding but involves destabilization of the closed conformation of the ion conduction gate. DHA lowers blood pressure in anesthetized wild-type but not in Slo1 knockout mice. DHA ethyl ester, contained in dietary supplements, fails to activate BK channels and antagonizes the stimulatory effect of DHA. Slo1 BK channels are thus receptors for long-chain omega-3 fatty acids, and these fatty acids—unlike their ethyl ester derivatives—activate the channels and lower blood pressure. This finding has practical implications for the use of omega-3 fatty acids as nutraceuticals for the general public and also for the critically ill receiving omega-3–enriched formulas.
Keywords: fish oil, lipids, KCa1.1, immunonutrition
High dietary intake of long-chain omega-3 polyunsaturated fatty acids such as docosahexaenoic acid (DHA) with a 22-carbon chain (for structure, see Fig. S1) is considered to promote good health in numerous and diverse ways, potentially protecting the immune, nervous, and cardiovascular systems (1, 2). Although large-scale clinical trials using dietary supplements have not yielded unequivocal results (3, 4), individuals are advised to consume fish rich in DHA for cardiovascular health (5) and numerous dietary supplements containing derivatives of omega-3 fatty acids are widely available. However, the precise mechanisms underlying the purported diverse health-promoting effects of DHA remain mostly unclear in part because molecular signaling pathways and effectors of the fatty acids are only beginning to be revealed (2, 6, 7). One such early study shows that DHA binds to and stimulates the G protein coupled receptor 120 (GPR120) with an EC50 of about 10 µM and this modulation contributes to the antiinflammatory effects of DHA (6). Because DHA may be enzymatically broken down to produce other biologically active molecules, DHA may exert multiple indirect effects (7).
Consumption of oily fish high in DHA such as anchovy, herring, mackerel, and salmon has been suggested to decrease blood pressure in some individuals (1, 8, 9). A diet supplemented with mackerel has been reported to lower blood pressure in healthy volunteers (10). Further, application of DHA dilates isolated blood vessels (7), potentially by activating large-conductance Ca2+-dependent K+ (Slo1 BK) channels (7, 11). In vascular smooth muscle cells (SMCs), Slo1 BK channels, which are allosterically activated by intracellular Ca2+ and depolarization (12), provide a pivotal vasodilatory influence (13, 14); activation of Slo1 BK channels acts to keep the membrane hyperpolarized, thus generally exerting a negative feedback influence on cellular excitability. Here we examined whether Slo1 BK channels are directly activated by DHA. We found that DHA but not its ethyl ester derivative potently and directly activates Slo1 BK channel complexes with the auxiliary subunit β1 in excised patches by destabilizing the closed conformation of the ion conduction gate. DHA lowers blood pressure without altering the heart or breathing rate in anesthetized wild-type mice but has no effect in Slo1 knockout mice. DHA ethyl ester, contained in dietary supplements, fail to alter Slo1 BK channels or blood pressure in mice. Thus, Slo1 BK channels are high-affinity receptors for omega-3 fatty acids involved in the blood-pressure lowering effect of the omega-3 polyunsaturated fatty acids.
Results
Injection of a bolus of DHA into a central vein in anesthetized wild-type mice substantially lowered the arterial blood pressure without altering the heart or breathing rate (Fig. 1 A, Left, and B and C) in a concentration-dependent manner (Fig. 1_C_). The transient nature of the hypotensive effect of DHA observed is probably due to diffusion and subsequent dilution of DHA in the whole circulatory system and also to a compensatory response of the animal elicited by the sudden drop in blood pressure. Because large-conductance Ca2+- and voltage-activated K+ (Slo1 BK) channels are important in regulation of vascular tone (13, 14), DHA may exert its hypotensive action by activating Slo1 BK channels. Consistent with this idea, injection of DHA into Slo1 knockout mice lacking BK channels (15) failed to alter blood pressure (Fig. 1_A_, Right, and B), clearly establishing the physiological importance of Slo1 BK channels in mediating the hypotensive effect of DHA. In SMCs, Slo1 BK channels contribute to outward K+ currents elicited by depolarization (15). Whole-cell outward currents recorded from dissociated aortic vascular SMCs from wild-type mice were enhanced by extracellular application of DHA (Fig. 1 D and F) but the current-enhancing effect of DHA was absent in the cells isolated from Slo1 knockout mice (Fig. 1 E and F). Enhanced activation of Slo1 BK channels in vascular SMCs by DHA thus underlies the hypotensive effect of DHA in wild-type mice.
Fig. 1.
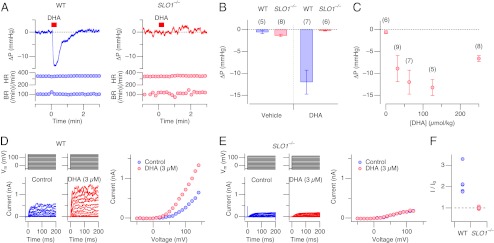
DHA lowers blood pressure in wild-type mice and enhances SMC BK channel currents. (A) Representative recordings of changes in arterial blood pressure (ΔP) , heart rate (HR), and breathing rate (BR) in wild-type (WT; Left) and Slo1 knockout (_SLO1_−/−; Right) mice by injection of DHA (62.5 μmol/kg) at the times indicated by the red bars. (B) Analysis of peak deflections of blood pressure in wild-type and _SLO1_−/− mice on injection of DHA and the vehicle (0.1% Tween-20 in 154 mM NaCl). Blood pressure values in WT mice before and after DHA injection were significantly different (P < 0.05) but no change was observed with the vehicle. In _SLO1_−/−, neither DHA nor the vehicle produced any change in blood pressure. (C) Dose dependence of the peak blood-pressure lowering effect of DHA. (D) Representative whole-cell currents (Left) from an isolated aortic SMC from a wild-type mouse elicited by voltage pulses before (blue) and after (red) application of DHA (3 µM). Peak current-voltage (IV) curves are also shown (Right). (E) Representative whole-cell currents (Left) and IV curves (Right) from an isolated aortic vascular SMC from a _SLO1_−/− mouse. (F) Fractional increase in the peak outward current size at 80 mV by DHA (3 µM for 4 min; see Fig. 2_D_) in WT and _SLO1_−/− mice. Fractional increases in the two groups are statistically different (P < 0.008). n = 5.
BK channels in SMCs are made of pore-forming Slo1 and auxiliary β1 subunits (16). Currents flowing through heterologously expressed BK channels formed by Slo1 and β1 (Slo1+β1) in cell-free inside-out patches elicited by depolarization in the absence of intracellular Ca2+ were rapidly and reversibly enhanced by DHA applied to the cytoplasmic side (Fig. 2 A and B). Application of DHA to the extracellular side was also effective but longer wash was required to reverse the effect (Fig. 2_C_). The finding that DHA robustly and reversibly activates Slo1+β1 channels in cell-free patches suggests that an intact intracellular signaling cascade is not essential but that DHA may directly bind to the channel complex. The current-enhancing effect of DHA applied to the cytoplasmic side was concentration dependent with an EC50 of ∼500 nM (Fig. 2_D_). This EC50 is less than typical serum concentrations of DHA derivatives after oral supplementation (17) and ∼20 times lower than the EC50 of DHA for GPR120, a G protein coupled receptor implicated in the antiinflammatory action of DHA (6).
Fig. 2.
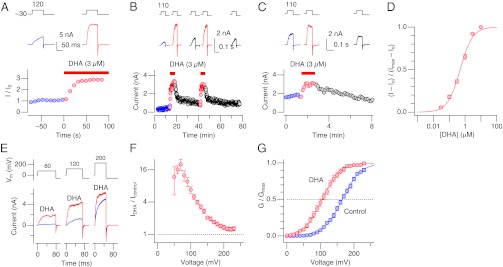
Characteristics of the DHA action in the absence of Ca2+ on Slo1+β1 channels expressed in HEK cells. (A) DHA rapidly increases currents through Slo1+β1 channels when applied to the cytoplasmic side. Currents at 120 mV were normalized to the average current size before DHA application and the peak outward current sizes are shown. Two representative currents are also shown (Top). (B) Current-enhancing effect of DHA applied to the intracellular side is reversible. Peak outward current sizes at 110 mV are plotted. Representative currents are also shown (Top). (C) DHA applied to the extracellular side in the outside-out configuration also increases currents through Slo1+β1 channels. (D) Concentration dependence. Currents were measured at 120 mV and every concentration of DHA shown was tested in each patch. n = 16. At 120 mV, DHA (3 µM) increases G/Gmax from ∼0.2 to ∼0.7 (Fig. 2_G_). The curve represents a Hill fit with EC50 = 486 ± 127 nM and the coefficient = 1.10 ± 0.28. (E) Representative currents through Slo1+β1 channels before and after application of DHA at three different voltages. (F) Fractional increases in current size at different voltages. n = 10. (G) Voltage dependence of normalized conductance (G/Gmax). Curves represent Boltzmann fits with _V_0.5 = 165.6 ± 0.9 mV and Q app = 0.90 ± 0.03 (control) and 105.37 ± 1.0 mV and 0.96 ± 0.03 (DHA). n = 10. Except in D, DHA was applied at 3 µM. All results were obtained without Ca2+.
DHA at 3 µM, a functionally saturating concentration (Fig. 2_D_), increased macroscopic Slo1+β1 currents by up to ∼20-fold, with greater fractional increases occurring at less depolarized voltages where open probability (Po) is normally low (Fig. 2 E and F). The increase in current by DHA was caused by a large shift in half-activation voltage (∆_V_0.5) required for activation of the channel complex by −59.0 ± 3.7 mV (10) with a small fractional increase in the steepness of voltage dependence (Qapp ratio = 1.09 ± 0.05 (10); Fig. 2_G_). The large ∆_V_0.5 by DHA is noticeably greater than those observed by application of cGMP-dependent protein kinase (18), which mediates the well-known vasodilatory influence of nitric oxide (19).
DHA markedly accelerated the macroscopic activation kinetics; the acceleration was particularly apparent at the voltages where the normalized conductance is ∼0.5, decreasing the time constant of activation by approximately threefold at 160 mV (Fig. 3 A_–_C). In contrast, the macroscopic deactivation kinetics at negative voltages was only slightly slowed by DHA (Fig. 3 A_–_C). The action of DHA did not require activation of the channel’s voltage sensors because DHA markedly increased single-channel Po at very negative voltages where the voltage sensors are mostly at rest (Fig. 3 D_–_F). Po at such negative voltages is primarily determined by the opening and closing transitions of the ion conduction gate of the channel (12), the latter of which controls the macroscopic deactivation kinetics. The absence of a noticeable effect of DHA on the deactivation kinetics at negative voltages (Fig. 3 A_–_C) indicates that DHA accelerates the opening of the ion conduction gate and increases Po.
Fig. 3.
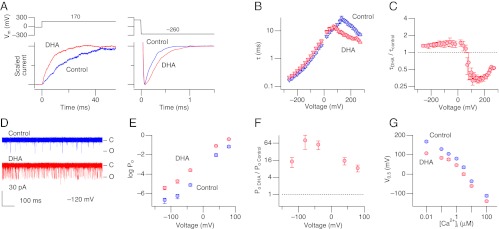
The stimulatory effect of DHA on Slo1+β1 channels does not require voltage-sensor activation or Ca2+ binding. (A) Representative scaled currents before (blue) and after (red) application of DHA at two different voltages to illustrate changes in kinetics. (B) Voltage dependence of the time constant of current relaxation of Slo1+β1 channels before (blue) and after (red) application of DHA. Time constant values were estimated from currents elicited by pulses to the voltages indicated from 0 or −30 mV (triangles) or from currents measured at the voltages indicated following pulses to 220 mV (inverse triangles). n = 6–10. (C) Fractional changes in the time constant of current relaxation by DHA. n = 6–10. (D) Single-channel openings at −120 mV before and after application of DHA without Ca2+. This patch contained ∼1,000 channels and 36 sweeps are shown superimposed. (E) Voltage dependence of single-channel Po in Slo1+β1 channels. n = 4–12. (F) Fractional increases in single-channel Po at different voltages by DHA. n = 4–12. (G) Ca2+ dependence of _V_0.5 without (blue) and with (red) DHA. The solution with EGTA but without added Ca2+ was assumed to have 10 nM [Ca2+]. n = 4–8. DHA was applied at 3 μM. Unless otherwise noted, all results were obtained without Ca2+.
DHA effectively activated Slo1+β1 channels and shifted _V_0.5 to the negative direction irrespective of the Ca2+ concentration (Fig. 3_G_), including ∼10 nM at which the Ca2+ sensors of Slo1 are unoccupied and also 100 µM at which the high-affinity Ca2+ sensors should be largely saturated with Ca2+ (20–22). However, the ∆_V_0.5 induced by DHA at 100 µM Ca2+ was smaller than that at 10 nM (P = 0.03), indicating a potential influence by Ca2+. DHA was also effective in activating Ca2+- and Mg2+-insensitive mutant Slo1 (D362A:D367A:E399A:∆894–895) (20, 21)+β1 channels (Fig. S2). The results collectively show that DHA promotes opening of the ion conduction gate of the Slo1 channel by destabilizing its closed conformation without any need for voltage-sensor activation or binding of Ca2+.
Omega-3 fatty acids have been suggested to have redox-modulating properties (23) but the effect of DHA on Slo1+β1 channels was unaltered by the membrane permeable reducing agent DTT or the oxidant chloramine-T (Fig. S3 A and B). Omega-3 fatty acids are metabolized by the enzyme cytochrome P450 epoxygenase, generating other metabolites that could in turn modulate native SMC BK channels (7). However, we found that the P450 epoxygenase inhibitor SKF525A (30 µM) did not alter the effect of DHA on Slo1+β1 channels expressed in human embryonic kidney (HEK) cells (Fig. S3 C and D).
Within the structure of DHA, both the aliphatic tail and the carboxylic acid head groups are important in activating Slo1+β1 channels because 17-hydroxyl DHA (17-OH DHA) and DHA ethyl ester (DHA EE) (Fig. S1) were without effect (Fig. 4_A_). In contrast with the large ∆_V_0.5 of −59.0 ± 3.7 mV (10) caused by DHA, DHA EE had no effect (∆_V_0.5 = −1.0 ± 6.4 mV) (8) (Fig. 4_B_). The negatively charged characteristic on the carboxylate moiety in the DHA head group, absent in DHA EE, may be essential for its effect in promoting opening of the ion conduction gate of the channel. Notably, when DHA and DHA EE were applied together, the resulting ∆_V_0.5 value was −31.8 ± 3.0 mV (9) (Fig. 4_B_), significantly smaller than that by DHA alone, approximately −60 mV. This observation suggests that DHA and DHA EE compete for the same sensor sites in the channel and that DHA EE is a competitive antagonist of DHA. In addition, DHA EE failed to increase whole-cell Slo1+β1 currents when applied to the extracellular side (Fig. S4). Eicosapentaenoic acid (EPA) is another omega-3 fatty acid with a 20-carbon chain (Fig. S1), 2 carbons shorter than that in DHA. EPA also increased currents through Slo1+β1 channels; however, the ∆_V_0.5 with EPA (−30.9 ± 3.1 mV) (11) was smaller than that by DHA. Like DHA EE, EPA ethyl ester (EPA EE; Fig. S1) was much less effective than EPA (Fig. 4_B_). These characteristics of DHA EE and EPA EE may have important practical implications for these are often found in dietary supplements (24); DHA EE and EPA EE in dietary supplements may interfere with the action of DHA and EPA obtained from natural foods. Whereas DHA and EPA are found abundantly in oily fish, α-linolenic acid (ALA) with an 18-carbon chain is concentrated in plant oils such as flaxseed oil (2). The ∆_V_0.5 by ALA was even smaller than that by EPA (−17.6 ± 2.7 mV; Fig. 4_B_). Comparison of the results from DHA, EPA, and ALA shows that the length of the fatty acid tail chain is another important determinant in activation of Slo1+β1 channels; marine-derived DHA with a 22-carbon chain is most effective among the three fatty acids.
Fig. 4.
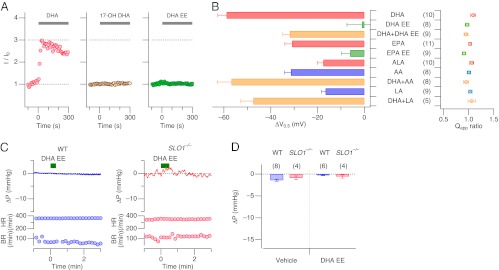
Differential effects of DHA and its derivatives on Slo1+β1 channels and mouse blood pressure. (A) 17-OH DHA (3 μM, Center) and DHA EE (3 μM, Right) are ineffective in enhancing Slo1+β1 currents compared with DHA (3 μM, Left). Scaled peak currents elicited by pulses to 120 mV without Ca2+ are plotted. n = 4 and 8 for 17-OH DHA and DHA EE, respectively. Up to 100 µM of DHA EE had no noticeable effect. (B) ∆_V_0.5 and fractional changes in Q app in Slo1+β1 channels by the compounds indicated. Results were obtained without Ca2+ and every compound was applied at 3 µM. Among the omega-3 fatty acids and their derivatives shown, the ∆_V_0.5 results for DHA, those for DHA+DHA EE and EPA, and those for DHA EE, EPA EE, and ALA constitute three statistically distinct groups after correcting for six-way combinatorial comparisons (P < 0.05). Among DHA and omega-6 fatty acids, the ∆_V_0.5 results for DHA, DHA+AA, and DHA+LA, those for AA, and those for LA form three statistically distinct groups after correcting for five-way combinatorial comparisons (P < 0.05). (C) Representative recordings of changes in arterial blood pressure (ΔP), heart rate (HR), and breathing rate (BR) in wild-type (WT; Left) and Slo1 knockout (_SLO1_−/−; Right) mice by injection of DHA EE (62.5 μmol/kg). (D) Analysis of peak deflections of blood pressure in wild-type and _SLO1_−/− mice upon injection of the vehicle alone (154 mM NaCl) or DHA EE (250 μmol/kg). None of the treatments produced a statistically significant change in blood pressure. DHA, docosahexaenoic acid; 17-OH DHA, 17-hydroxyl docosahexaenoic acid; DHA EE, docosahexaenoic acid ethyl ester; EPA, eicosapentaenoic acid; EPA EE, eicosapentaenoic acid ethyl ester; ALA, α-linolenic acid; AA, arachidonic acid; and LA, linoleic acid. See Fig. S1 for structure.
Some studies have postulated the importance of the omega-6/omega-3 fatty acid ratio in disease prevention and good health (25). Arachidonic acid (AA), an omega-6 fatty acid with a 20-carbon chain with four double C-C bonds (Fig. S1), plays key roles in a variety of physiological phenomena, including inflammation and vasodilation (26). AA stimulated Slo1+β1 channels but the ∆_V_0.5 by AA (−31.3 ± 2.7 mV) was markedly smaller than that by DHA (Fig. 4_B_). Linoleic acid (LA), a shorter omega-6 fatty acid, produced an even smaller response (∆_V_0.5 = −17.0 ± 1.7 mV; Fig. 4_B_). When the omega-3 fatty acid DHA and the omega-6 fatty acid AA were applied together, the resulting ∆_V_0.5 was large (−57.7 ± 6.1 mV) and indistinguishable from that by DHA alone. Similarly, the ∆_V_0.5 with DHA and LA together (−47.4 ± 5.3 mV) was indistinguishable from that with DHA alone.
The inability of DHA EE to activate Slo1+β1 channels predicts that DHA EE should have no effect on whole-animal blood pressure. Indeed, injection of a bolus of DHA EE produced no change in blood pressure in wild-type and also in _SLO1_−/− mice (Fig. 4 C and D), further supporting the physiological relevance of modulation of BK channels by DHA.
Discussion
Here we have clearly demonstrated that marine-derived long-chain omega-3 fatty acids such as DHA activate vascular Slo1 BK channels and lower blood pressure. The stimulatory action of these fatty acids has a rapid onset, is reversible, and is observed in cell-free patches. We thus suggest that the fatty acids directly bind to and stimulate Slo1 BK channels.
Our in vivo observations of a direct effect of omega-3 fatty acids but not of their ester derivatives on blood pressure may have direct clinical implications for the use of so-called “immunonutrition”, formulas enriched in some amino acids and/or omega-3 fatty acids, for critically ill patients. These emulsions, which are available for enteral as well as for parenteral (central venous) application, may result in administration of up to 10 g of DHA/EPA per patient per day and may contain either free or ester-conjugated omega-3 fatty acids (27). Thus, application doses mimicking our animal model at which significant blood pressure-lowering effects of DHA were observed might be achieved in the clinical context. Immunomodulating diets containing fish oil might favorably affect outcome in sepsis and adult respiratory distress syndrome (27). However, there is evidence to suggest caution regarding early implementation of parenteral and in particular immunomodulating diets (28, 29) in these patients. Although the mechanisms negatively affecting outcome in the early course of critical illness have not been studied in detail for parenteral nutrition, they are typically associated with development of hypotension, shock, and cardiovascular collapse.
The stimulatory effect of DHA on the BK channel is observable at ≤100 nM. This strikingly differs from the effects of DHA on other channel types for which typically inhibitory effects at higher concentrations, sometimes ≥100 µM, were reported (30–32). Previous studies reported a stimulatory effect of DHA on native BK channel currents (7, 11); however, whether DHA acts directly on the channels remained unresolved. We have established that DHA rapidly and reversibly stimulates Slo1 BK channels in excised patches. This finding suggests that DHA directly binds to the Slo1 channel complex; the channel complex itself may be a high-affinity receptor for DHA. However, the conclusive proof of this postulate will await identification of the binding site for DHA within the channel complex. A previous study using BK channels expressed in Xenopus oocytes reported direct modulation of molecularly distinct BK channel complexes by arachidonic acid and DHA but at higher concentrations (≥20 µM) (33). Shorter lipids were previously tested on native BK channels and found to have diverse effects (34). The current-enhancing effect of DHA on Slo1+β1 channels in mammalian cells described here is robust and occurs at much lower concentrations of DHA. Additionally, the concentration required to activate the BK channel is ∼20 times lower than that required to stimulate GPR120 involved in the antiinflammatory action of DHA (6). DHA is released from the plasma membrane by the G protein activated Ca2+-dependent phospholipase A2 (35, 36). Therefore, stimulation of BK channels by DHA, a previously unexplored modulatory pathway, has strong potential to contribute to a variety of physiological and pathophysiological processes. Importantly and practically, the effectiveness of marine-derived DHA and EPA but not their ethyl ester derivatives found in dietary supplements (24) underscores the importance of intake of omega-3 fatty acids from natural foods.
Materials and Methods
Electrophysiology and Analysis.
Human Slo1 (KCNMA1, AAB65837) and the auxiliary subunit β1 (KCNMB1, NP_004128) were transiently transfected into HEK cells using FuGene 6 (Roche) (37). In many of the experiments, β1 with EGFP fused to the C terminus (β1-EGFP) was used and the results obtained with β1 and β1-EGFP were indistinguishable. The electrophysiological measurements were performed 36–48 h after transfection as described previously (38). To record native BK channel currents, mice were euthanized with CO2 following an approved protocol and aortic vascular smooth muscle cells were isolated as described (37) except that the enzymatic digestion durations were doubled. Ionic currents in cell-free patches were recorded under a symmetrical K+ condition. The external solution contained (in millimolars): 140 KCl, 2 MgCl2, 10 Hepes, pH 7.2 with _N_-methyl-D-glucamine (NMG). The internal solution without Ca2+ contained (in millimolars): 140 KCl, 11 EGTA, 0.02 18C6TA, 10 Hepes, pH 7.2 with NMG. Internal solutions with greater concentrations of free Ca2+ contained various amounts of EGTA or HEDTA and added CaCl2 as appropriate (39). For whole-cell current measurements, the external solution contained (in millimolars): 134 NaCl, 6 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, 10 Hepes, pH 7.4 (with NMG). The intracellular solution contained (in millimolars): 110 K aspartate, 30 KCl, 10 NaCl, 2 MgCl2, 10 Hepes, pH 7.2 (with NMG). All electrophysiological experiments were performed at room temperature. The results were analyzed using custom routines written in IgorPro (WaveMetrics). Normalized conductance-voltage (G/Gmax) curves were constructed as described (38) from extrapolated tail current sizes and fitted with a Boltzmann function so that changes in voltage dependence of activation were described by changes in half-activation voltage (∆_V_0.5) and the fractional change in apparent charge movement (Q app ratio). Estimated equation parameters are presented as mean ± 95% confidence interval. In some patches, the effect of DHA diminished with time and these results were not included in our analysis.
Reagents.
DHA were obtained from Sigma and Avanti and they produced indistinguishable results. DHA EE, 17-OH DHA, EPA, and EPA EE were purchased from Cayman. AA, ALA, and LA were from Sigma. The stock solutions in ethanol were stored at −20 °C and diluted to the final concentrations immediately before use by vortexing. The vehicle at the concentrations used for DHA, DHA EE, EPA, EPA EE, AA, ALA, and LA (<0.01%) had no effect. For the experiments with 17-OH DHA, the vehicle concentration was higher (1%) and for those experiments, the control condition also contained the same concentration of ethanol.
Mouse Experiments.
Mouse blood pressure experiments were carried out in accordance with the recommendations of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and in strict compliance with the German legislation regarding animal protection after protocols were approved by the local government authorities (Thuringian State Offices for Consumer Protection and Food Safety; 02-035/10). FVB/NJ mice (wild-type and Slo1 knockout) were obtained from Meredith et al. (15). Animals (9–12 wk of age) were housed on a 12/12 h light/dark cycle with the light cycle beginning at 6:00 AM. All mice had ad libitum access to rodent chow and water until the experiment. Mice were anesthetized with ketamine/xylazine (100 mg/kg and 8 mg/kg body weight, respectively). Sterile catheters (polyethylene, outer diameters of 480 µm for the carotid artery and 960 µm for the vein) were inserted after midline skin incision for continuous blood pressure monitoring (pressure transducer TBD-1222; Föhr Medical Instruments) or application of DHA and its derivatives. After the experiment, animals were killed by an overdose of the anesthetic. Blood pressure results were sampled at 100 Hz and processed through a digital binomial filter. The heart and breathing rates were determined by examining unfiltered fluctuations in blood pressure in segments of 1,024 data points with a fast Fourier transform followed by fitting with Gaussian curves using IgorPro.
Statistics.
The numbers of measurements are indicated in the figures. Statistical results are presented in the text as mean ± SEM (n), where n is the number of independent measurements. Statistical comparisons were made using the Mann–Whitney or Wilcoxon test with an α level of 0.05, which was corrected for desired multiple comparisons using the Bonferroni method when appropriate.
Supplementary Material
Supporting Information
Acknowledgments
We thank Profs. Meredith and Aldrich for the Slo1 knockout mice. This work was supported by the public in part through the National Institutes of Health (R01GM057654), the Federal Ministry of Research and Education within the framework of the Center for Sepsis Control and Care (BMBF 01 EO 1002), the German Research Foundation (DFG FOR 1738), the National Natural Science Foundation of China (31271217), and the Key Project of Shanghai Science and Technology Commission (Contract 11JC1406400).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Saravanan P, Davidson NC, Schmidt EB, Calder PC. Cardiovascular effects of marine omega-3 fatty acids. Lancet. 2010;376(9740):540–550. doi: 10.1016/S0140-6736(10)60445-X. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58(20):2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 3.Jicha GA, Markesbery WR. Omega-3 fatty acids: Potential role in the management of early Alzheimer’s disease. Clin Interv Aging. 2010;5:45–61. doi: 10.2147/cia.s5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kromhout D, Yasuda S, Geleijnse JM, Shimokawa H. Fish oil and omega-3 fatty acids in cardiovascular disease: Do they really work? Eur Heart J. 2012;33(4):436–443. doi: 10.1093/eurheartj/ehr362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kris-Etherton PM, Harris WS, Appel LJ. American Heart Association. Nutrition Committee Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106(21):2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 6.Oh DY, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142(5):687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang RX, Chai Q, Lu T, Lee HC. Activation of vascular BK channels by docosahexaenoic acid is dependent on cytochrome P450 epoxygenase activity. Cardiovasc Res. 2011;90(2):344–352. doi: 10.1093/cvr/cvq411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu JC, Conklin SM, Manuck SB, Yao JK, Muldoon MF. Long-chain omega-3 fatty acids and blood pressure. Am J Hypertens. 2011;24(10):1121–1126. doi: 10.1038/ajh.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramel A, Martinez JA, Kiely M, Bandarra NM, Thorsdottir I. Moderate consumption of fatty fish reduces diastolic blood pressure in overweight and obese European young adults during energy restriction. Nutrition. 2010;26(2):168–174. doi: 10.1016/j.nut.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Singer P, et al. Lipid and blood pressure-lowering effect of mackerel diet in man. Atherosclerosis. 1983;49(1):99–108. doi: 10.1016/0021-9150(83)90011-4. [DOI] [PubMed] [Google Scholar]
- 11.Lai LH, et al. Effects of docosahexaenoic acid on large-conductance Ca2+-activated K+ channels and voltage-dependent K+ channels in rat coronary artery smooth muscle cells. Acta Pharmacol Sin. 2009;30(3):314–320. doi: 10.1038/aps.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horrigan FT, Aldrich RW. Coupling between voltage sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels. J Gen Physiol. 2002;120(3):267–305. doi: 10.1085/jgp.20028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patterson AJ, Henrie-Olson J, Brenner R. Vasoregulation at the molecular level: A role for the β1 subunit of the calcium-activated potassium (BK) channel. Trends Cardiovasc Med. 2002;12(2):78–82. doi: 10.1016/s1050-1738(01)00146-3. [DOI] [PubMed] [Google Scholar]
- 14.Nelson MT, Bonev AD. The β1 subunit of the Ca2+-sensitive K+ channel protects against hypertension. J Clin Invest. 2004;113(7):955–957. doi: 10.1172/JCI21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem. 2004;279(35):36746–36752. doi: 10.1074/jbc.M405621200. [DOI] [PubMed] [Google Scholar]
- 16.Brenner R, et al. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature. 2000;407(6806):870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 17.Kim W, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids—physiological relevance of dose. Prostaglandins Leukot Essent Fatty Acids. 2010;82(4-6):155–158. doi: 10.1016/j.plefa.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schubert R, Nelson MT. Protein kinases: Tuners of the BKCa channel in smooth muscle. Trends Pharmacol Sci. 2001;22(10):505–512. doi: 10.1016/s0165-6147(00)01775-2. [DOI] [PubMed] [Google Scholar]
- 19.Archer SL, et al. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc Natl Acad Sci USA. 1994;91(16):7583–7587. doi: 10.1073/pnas.91.16.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia XM, Zeng X, Lingle CJ. Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature. 2002;418(6900):880–884. doi: 10.1038/nature00956. [DOI] [PubMed] [Google Scholar]
- 21.Zhang G, et al. Ion sensing in the RCK1 domain of BK channels. Proc Natl Acad Sci USA. 2010;107(43):18700–18705. doi: 10.1073/pnas.1010124107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sweet TB, Cox DH. Measuring the influence of the BKCa β1 subunit on Ca2+ binding to the BKCa channel. J Gen Physiol. 2009;133(2):139–150. doi: 10.1085/jgp.200810129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Nunzio M, Valli V, Bordoni A. Pro- and anti-oxidant effects of polyunsaturated fatty acid supplementation in HepG2 cells. Prostaglandins Leukot Essent Fatty Acids. 2011;85(3-4):121–127. doi: 10.1016/j.plefa.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Neubronner J, et al. Enhanced increase of omega-3 index in response to long-term n-3 fatty acid supplementation from triacylglycerides versus ethyl esters. Eur J Clin Nutr. 2011;65(2):247–254. doi: 10.1038/ejcn.2010.239. [DOI] [PubMed] [Google Scholar]
- 25.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 2008;233(6):674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 26.Imig JD. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol Rev. 2012;92(1):101–130. doi: 10.1152/physrev.00021.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marik PE, Zaloga GP. Immunonutrition in critically ill patients: A systematic review and analysis of the literature. Intensive Care Med. 2008;34(11):1980–1990. doi: 10.1007/s00134-008-1213-6. [DOI] [PubMed] [Google Scholar]
- 28.Bertolini G, et al. Early enteral immunonutrition in patients with severe sepsis: Results of an interim analysis of a randomized multicentre clinical trial. Intensive Care Med. 2003;29(5):834–840. doi: 10.1007/s00134-003-1711-5. [DOI] [PubMed] [Google Scholar]
- 29.Casaer MP, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365(6):506–517. doi: 10.1056/NEJMoa1102662. [DOI] [PubMed] [Google Scholar]
- 30.Wilding TJ, Chen K, Huettner JE. Fatty acid modulation and polyamine block of GluK2 kainate receptors analyzed by scanning mutagenesis. J Gen Physiol. 2010;136(3):339–352. doi: 10.1085/jgp.201010442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guizy M, Arias C, David M, González T, Valenzuela C. Ω-3 and Ω-6 polyunsaturated fatty acids block HERG channels. Am J Physiol Cell Physiol. 2005;289(5):C1251–C1260. doi: 10.1152/ajpcell.00036.2005. [DOI] [PubMed] [Google Scholar]
- 32.Börjesson SI, Hammarström S, Elinder F. Lipoelectric modification of ion channel voltage gating by polyunsaturated fatty acids. Biophys J. 2008;95(5):2242–2253. doi: 10.1529/biophysj.108.130757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun X, Zhou D, Zhang P, Moczydlowski EG, Haddad GG. β-subunit-dependent modulation of hSlo BK current by arachidonic acid. J Neurophysiol. 2007;97(1):62–69. doi: 10.1152/jn.00700.2006. [DOI] [PubMed] [Google Scholar]
- 34.Clarke AL, Petrou S, Walsh JV, Jr, Singer JJ. Modulation of BKCa channel activity by fatty acids: Structural requirements and mechanism of action. Am J Physiol Cell Physiol. 2002;283(5):C1441–C1453. doi: 10.1152/ajpcell.00035.2002. [DOI] [PubMed] [Google Scholar]
- 35.Strokin M, Sergeeva M, Reiser G. Docosahexaenoic acid and arachidonic acid release in rat brain astrocytes is mediated by two separate isoforms of phospholipase A2 and is differently regulated by cyclic AMP and Ca2+ Br J Pharmacol. 2003;139(5):1014–1022. doi: 10.1038/sj.bjp.0705326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia MC, Kim HY. Mobilization of arachidonate and docosahexaenoate by stimulation of the 5-HT2A receptor in rat C6 glioma cells. Brain Res. 1997;768(1-2):43–48. doi: 10.1016/s0006-8993(97)00583-0. [DOI] [PubMed] [Google Scholar]
- 37.Hou S, et al. Bilirubin oxidation end products directly alter K+ channels important in the regulation of vascular tone. J Cereb Blood Flow Metab. 2011;31(1):102–112. doi: 10.1038/jcbfm.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horrigan FT, Heinemann SH, Hoshi T. Heme regulates allosteric activation of the Slo1 BK channel. J Gen Physiol. 2005;126(1):7–21. doi: 10.1085/jgp.200509262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hou S, Xu R, Heinemann SH, Hoshi T. Reciprocal regulation of the Ca2+ and H+ sensitivity in the SLO1 BK channel conferred by the RCK1 domain. Nat Struct Mol Biol. 2008;15(4):403–410. doi: 10.1038/nsmb.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information