Incubation Periods of Mosquito-Borne Viral Infections: A Systematic Review (original) (raw)
Abstract
Mosquito-borne viruses are a major public health threat, but their incubation periods are typically uncited, non-specific, and not based on data. We systematically review the published literature on six mosquito-borne viruses selected for their public health importance: chikungunya, dengue, Japanese encephalitis, Rift Valley fever, West Nile, and yellow fever viruses. For each, we identify the literature's consensus on the incubation period, evaluate the evidence for this consensus, and provide detailed estimates of the incubation period and distribution based on published experimental and observational data. We abstract original data as doubly interval-censored observations. Assuming a log-normal distribution, we estimate the median incubation period, dispersion, 25th and 75th percentiles by maximum likelihood. We include bootstrapped 95% confidence intervals for each estimate. For West Nile and yellow fever viruses, we also estimate the 5th and 95th percentiles of their incubation periods.
Introduction
Mosquito-borne viruses are a major public health threat. Dengue virus (DENV), endemic in tropical settings, has recently spread to more temperate climes, causing an estimated 50–100 million infections and 12,500 deaths per year.1 Similarly, epidemics of West Nile virus (WNV) are a growing concern—a 2012 epidemic in the United States caused 5,674 reported cases (51% of them neuroinvasive) and 286 deaths.2 Knowledge of the incubation period (the time between infection and the onset of symptoms) would improve 1) estimation of the timing, and hence the probable location, of infection;3,4 2) accurate modeling of the disease process using predictive models; and 3) evaluation of control measures (including quarantine) targeting symptomatic individuals.5 However, the incubation periods of many mosquito-borne viruses are typically uncited, non-specific, and not based on data.6,7 With the previously mentioned gaps in mind, we systematically reviewed the published literature on six mosquito-borne viruses selected for their public health importance: chikungunya virus (CHIKV), DENV, Japanese encephalitis virus (JEV), Rift Valley fever virus (RVFV), WNV, and yellow fever virus (YFV). For each virus, we aim to 1) identify the literature's consensus on the incubation period, 2) evaluate the evidence for this consensus, and 3) provide estimates of the incubation period that contain detail on the distribution based on published experimental and observational data.
Materials and Methods
Search, assessment, data abstraction, and analyses largely followed the methods of Lessler and others.6
Search strategy and selection criteria.
Searches were conducted using PubMed, Google Scholar, and ISI Web of Knowledge 4.0 as described in Lessler and others.6 Searches were conducted between April 15, 2010 and January 6, 2011, with no restrictions on the earliest date of the articles returned. Each search was done with common variations of the virus name, specifically: Japanese encephalitis, Japanese B Encephalitis, JE, JEV, JBE, West Nile, WN, WNV, Rift Valley fever, RVF, RVFV, chikungunya, CHIK, CHIKV, dengue, DEN, DENV, yellow fever, YF, and YFV. We also reviewed the infectious disease reference, Field's Virology, several library catalogues, and the Cochrane Library.
Two reviewers independently reviewed and categorized abstracts. Abstracts summarizing a study of human infection of one of the six mosquito-borne viruses included in this review were designated for full-text review. The reviewers resolved discrepancies through discussion and consensus. This review satisfies the PRISMA and QUORUM systematic review checklists.
Assessment.
Assessment was performed on documents included in the full-text review as described in Lessler and others.6 Documents were classified as either containing a statement of the incubation period or not. Those containing an incubation period statement were further classified according to whether the statement was 1) based on and ascertainable from original data, 2) based on but not ascertainable from original data, 3) sourced, or 4) unsourced. Those not containing an incubation period statement were further classified according to whether the article 1) contained original data that could be used to ascertain an incubation period, 2) contained original data that could not be used to ascertain an incubation period, or 3) did not contain any original data.
Two reviewers abstracted incubation period statements and original incubation period data as described in Lessler and others.6 We report the incubation period range consistent with over 50% of the abstracted statements.
Pooled analysis.
As in Lessler and others,6 original data that could be used to ascertain an incubation period were abstracted as doubly interval-censored observations. Assuming a log-normal distribution, incubation period quantiles and a dispersion parameter were estimated for each virus by maximum-likelihood using the coarseDataTools package for R.8 We used 500 bootstrapped samples to calculate 95% confidence intervals (CIs). For each of DENV, WNV, and YFV, pooled data were analyzed 1) including only those who were infected by a mosquito, and 2) including all abstracted cases. For each of CHIKV, JEV, and RVFV, pooled data were analyzed including all abstracted cases, because there were not enough mosquito-infection observations to perform separate analyses. Cases of maternal-child transmission were excluded from the analyses. We conducted a separate analysis of cases infected by WNV through blood transfusion or surgery. All analyses were done using the R statistical package (version 2.14.1). All data and a complete bibliography are available from the authors upon request.
Results
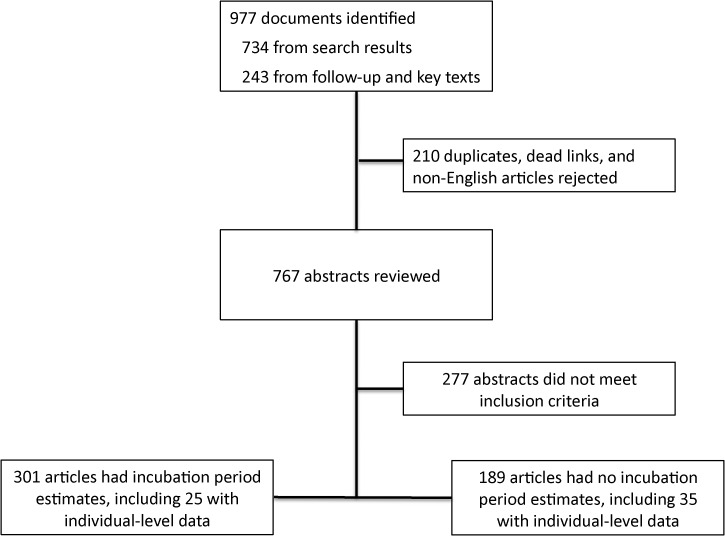
We identified 977 articles containing incubation period statements (Figure 1). Table 1 summarizes the incubation periods stated in the literature for each virus. Of the 375 estimates included in these articles, 34 (9%) were original, 129 (34%) were not original but provided a source, and 212 (57%) were not original and did not provide a source. Table 2 summarizes the 60 articles containing individual-level data appropriate for pooled analysis (Table 2). Estimates for the incubation period of YFV and DENV had the most support (14 studies and 19 studies, respectively). Fewer than 25 observations were available for each of CHIKV, RVFV, and WNV; and only 6 observations were available for JEV.
Figure 1.
Systematic review process.
Table 1.
Summary of incubation period estimates in the published literature
| Disease | Literature estimates (days) | Number of estimates (%) | Participants in experimental studies | |||
|---|---|---|---|---|---|---|
| Range | Central tendency | Unsourced estimates | Sourced estimates | Original estimates [experimental/ observational] | ||
| CHIKV | 2–10 | 3 | 21 (60%) | 12 (34%) | 2 [0/2] | N/A |
| DENV | 3–10 | 4 | 66 (49%) | 56 (41%) | 13 [8/5] | 128 |
| JEV | 5–15 | 7* | 25 (69%) | 9 (25%) | 2 [0/2] | N/A |
| RVFV | 3–6 | 3–4† | 10 (59%) | 3 (18%) | 4 [0/4] | 1 |
| WNV | 3–14 | 4‡ | 51 (58%) | 32 (36%) | 5 [1/4] | 8 |
| YFV | 3–6 | 6 | 39 (61%) | 17 (27%) | 8 [1/7] | 25 |
Table 2.
Details of works that included individual-level data for inclusion in the pooled analysis*
| Location | Study type | N | Population | Infection mechanism | |
|---|---|---|---|---|---|
| CHIKV | |||||
| ICDDR, 200913 | Bangladesh | Observational | 2 | Male in 30s, 36-year-old female | Mosquito |
| CDC, 200614 | Zimbabwe | Observational | 1 | Adult male | Mosquito |
| Parola and others, 200615 | Indian Ocean Islands | Observational | 1 | 60-year-old female | Direct contact with blood |
| Beltrame and others, 200716 | Italy | Observational | 13 | 4 females ages 36–57, 9 males ages 31–66 | Mosquito |
| Receveur and others, 201017 | Maldives Islands (Indian Ocean) | Observational | 1 | Male in 30s | Mosquito |
| Tang, 200618 | Mauritius (Indian Ocean) | Observational | 1 | 66-year-old male | Mosquito |
| Soon and others, 200719 | Southern Indian | Observational | 1 | 49-year-old female | Mosquito |
| Volpe and others, 200620 | Reunion Island | Observational | 1 | 46-year-old female | Mosquito |
| DENV | |||||
| Ashburn and others, 190721 | Philippines | Experimental | 10 | Adult males | Injection |
| Bancroft, 190622 | Australia | Experimental | 2 | Males ages 29, 34 | Mosquito |
| Cleland and others, 191923 | Australia | Experimental | 11 | 28-year-old female; 10 males ages 25–76 | Injection |
| Yeo and others, 200524 | Vietnam | Observational | 1 | 25-year-old male | Mosquito |
| Cleland and others, 191825 | Australia | Experimental | 17 | 27-year-old female; 16 males ages 18–56 | injection |
| Ito and others, 200726 | Japan | Observational | 22 | Not reported | Mosquito |
| Senanayake, 200627 | Australia | Observational | 1 | Female in 30s | Mosquito |
| Courtney and others, 200928 | Guatemala | Observational | 1 | 15-year-old male | Mosquito |
| Hanna and others, 200329 | Australia | Observational | 1 | Adult male | Mosquito |
| Hill, 199230 | Haiti | Observational | 1 | 31-year-old female | Mosquito |
| Graham, 190331 | Lebanon | Experimental; Observational | 8 | 7 adult males; 1 adult female | Mosquito |
| Siler and others, 192632 | Philippines | Experimental | 47 | Adult males | Mosquito |
| Simmons and others, 193133 | Philippines | Experimental | 24 | Adult males | Mosquito; Injection |
| Schule, 192834 | Philippines | Experimental | 9 | Males ages 18–27 | Mosquito |
| Chandler and others, 192335 | United States | Observational | 6 | 4 males ages 20–30; 2 females ages 19,31 | Mosquito; Injection |
| Hare, 189836 | Australia | Observational | 2 | Adults | Mosquito |
| Koizumi and others, 191837 | Taiwan | Observational; Experimental | 2 | Adults | Mosquito |
| Agramonte, 190638 | Cuba | Observational | 2 | Adolescent males | Mosquito |
| Vassal and others, 190939 | Boat in Indian Ocean | Observational | 2 | Males | Mosquito |
| JEV | |||||
| Buhl and others, 199640 | Denmark | Observational | 1 | 51-year-old male | Mosquito |
| Caramello and others, 200741 | Italy | Observational | 1 | 49-year-old male | Mosquito |
| Lehtinen and others, 200842 | Finland | Observational | 1 | 60-year-old male | Mosquito |
| MacDonald and others, 198943 | Australia | Observational | 1 | 10-year-old female | Mosquito |
| Ostlund and others, 200444 | Sweden | Observational | 1 | 80-year-old male | Mosquito |
| Wittesjo and others, 199545 | Sweden | Observational | 1 | 60-year-old female | Mosquito |
| RVFV | |||||
| Daubney and others, 193146 | Kenya | Experimental | 1 | Adult male | Inoculation |
| Findlay, 193247 | UK | Observational | 3 | Adult males | Contact with infected animal tissue |
| Francis and others, 193548 | USA | Observational | 1 | 22-year-old male | Aerosol when cleaning laboratory |
| Hoogstraal and others, 197911 | Egypt | Observational | 6 | Adults | Contact with infected animal tissue |
| Kitchen, 193449 | USA | Observational | 2 | Males ages 24, 37 | Laboratory infection |
| Mundel and others, 195110 | South Africa | Observational | 6 | Males ages 32–64 | Contact with infected animal tissue |
| Sabin and others, 194750 | USA | Observational | 1 | Adult male | Laboratory infection |
| Smithburn and others, 194951 | Uganda | Observational | 3 | Males ages 22–25 | Laboratory infection |
| WNV | |||||
| Campbell and others, 200252 | USA | Observational | 2 | Adults | Laboratory infection |
| Kokernot and others, 195912 | South Africa | Observational | 1 | 26-year-old male | Mosquito |
| Nash and others, 200153 | Airplane | Observational | 1 | Adult | Mosquito |
| Olejnik, 195254 | Israel | Observational | 6 | Adults and children | Mosquito |
| Southam and others, 195455 | USA | Experimental | 8 | Males and females ages 23–73 | Inoculation |
| YFV | |||||
| Hindle, 193056 | United States | Observational | 2 | Adult males | Direct contact with blood |
| Colebunders and others, 200257 | The Gambia | Observational | 1 | 47-year-old female | Mosquito |
| Smith and others, 196858 | Cuba | Observational | 31 | Males and females, ages 6–60 | Mosquito |
| McFarland and others, 199759 | Brazil | Observational | 1 | 45-year-old male | Mosquito |
| CDC, 200060 | Venezuela | Observational | 1 | 48-year-old male | Mosquito |
| Low and others, 193161 | London | Observational | 1 | 28-year-old male | Laboratory infection |
| Carter, 190162 | USA | Observational | 18 | 16 males, 2 females | Mosquito |
| Reed, 190263 | Cuba | Experimental | 25 | Not reported | Mosquito; injection |
| Berry and others, 193164 | USA | Observational | 2 | Males ages 35, 24 | Laboratory infection |
| Bugher and others, 194465 | Columbia | Observational | 1 | Male | Mosquito |
| Dudley, 193366 | Sierra Leone | Observational | 4 | Adult males | Mosquito |
| WHO, 200067 | Venezuela | Observational | 1 | 48-year-old male | Mosquito |
| CDC, 200268 | Brazil | Observational | 1 | 47-year-old male | Mosquito |
| Nolla-Salas and others, 198969 | West Africa | Observational | 1 | 37-year-old female | Mosquito |
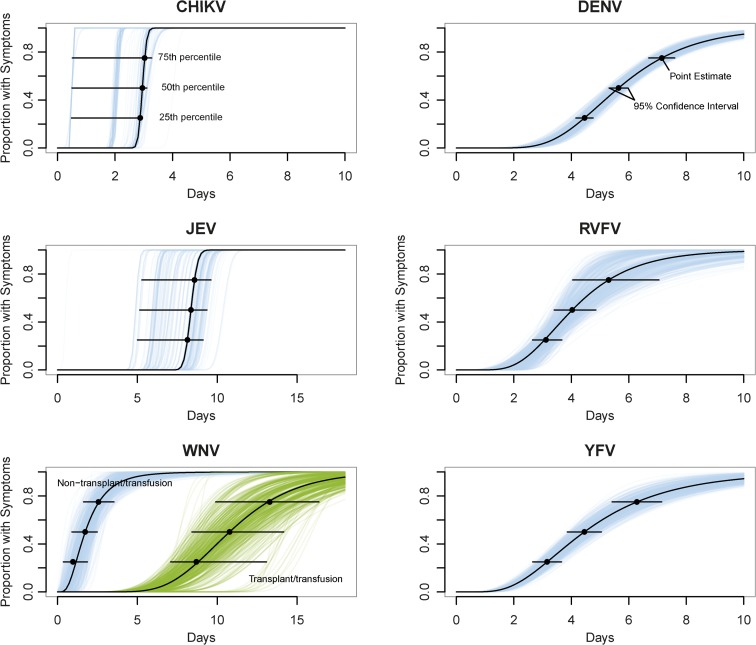
Estimates of the full distribution of each incubation period using pooled data are shown in Figure 2 and Table 3. To characterize the distribution, we provide the incubation periods within which 25%, 50%, and 75% of cases become symptomatic. Where possible, we provide incubation period estimates for the subset of mosquito-acquired infections. We also provide the 5th and 95th percentile incubation period estimates for WNV (all observations) and YFV (all observations and the mosquito-acquired subset). There were insufficient data to confidently estimate the 5th or 95th incubation period percentiles for CHIKV, DENV, JEV, and RVFV. Median incubation periods ranged from 2.6 days (for WNV) to 8.4 days (for JEV). Dispersions ranged from 1.04 to 1.82.
Figure 2.
Estimated cumulative distributions of the incubation periods. Horizontal bars show the 95% confidence intervals at the 25th, 50th, and 75th percentiles. Individual lines represent bootstrap samples.
Table 3.
Percentiles of the time of symptom onset and dispersion for incubation period distributions
| Virus | Estimate (95% CI) of time of symptom onset (days)* | Dispersion (95% CI) | ||||
|---|---|---|---|---|---|---|
| 5th | 25th | 50th | 75th | 95th | ||
| CHIKV | N/A | 2.9 (0.5, 3.0) | 3.0 (0.5, 3.1) | 3.0 (0.5, 3.3) | N/A | 1.04 (1.04, 1.08) |
| DENV | N/A | 4.5 (4.1, 4.9) | 5.6 (5.3, 6.0) | 7.1 (6.7, 7.6) | N/A | 1.41 (1.34, 1.50) |
| DENV mosquito only | N/A | 4.3 (3.8, 4.7) | 5.3 (5.0, 5.7) | 6.6 (6.2, 7.2) | N/A | 1.37 (1.27, 1.52) |
| JEV | N/A | 8.1 (5.0, 9.1) | 8.4 (5.1, 9.4) | 8.6 (5.3, 9.6) | N/A | 1.04 (1.04, 1.05) |
| RVFV | N/A | 3.1 (2.7, 3.7) | 4.0 (3.4, 4.9) | 5.3 (4.0, 7.1) | N/A | 1.50 (1.22, 1.82) |
| WNV | 1.0 (0.4, 1.9) | 1.7 (0.9, 2.5) | 2.6 (1.6, 3.5) | 3.8 (2.5, 5.6) | 7.0 (3.6, 12.4) | 1.82 (1.27, 2.67) |
| WNV mosquito only | N/A | 2.8 (0.4, 3.0) | 2.9 (0.5, 3.1) | 3.0 (0.6, 3.2) | N/A | 1.04 (1.04, 1.29) |
| WNV transplant only | N/A | 8.7 (7.1, 13.1) | 10.8 (8.4, 14.2) | 13.3 (9.9, 16.4) | N/A | 1.35 (1.12, 1.47) |
| YFV | 1.9 (1.7, 2.3) | 3.2 (2.8, 3.5) | 4.4 (4.0, 5.0) | 6.3 (5.3, 7.3) | 10.3 (7.9, 12.8) | 1.66 (1.48, 1.82) |
| YFV mosquito only | 1.9 (1.6, 2.3) | 3.1 (2.8, 3.5) | 4.4 (3.9, 5.0) | 6.2 (5.1, 7.4) | 10.3 (7.5, 13.1) | 1.67 (1.47, 1.84) |
Chikungunya virus.
The CHIKV is a mosquito-borne virus first discovered in Tanzania in the early 1950s, and it causes an illness characterized by fever and joint pain.70 Historically, infection rates were low and limited to regions in Africa and Asia.71 However, a recent mutation may have increased the virus' preference for the Aedes (Ae.) albopictus mosquito.72,73 Aedes albopictus is thought to have been a major vector in recent outbreaks in India, several countries in the Indian Ocean,74 and the first European outbreak in Northern Italy in 2007.75 The continued spread of Ae. albopictus across Europe and the Americas heightens the potential public health impact of the virus, for which there is no vaccine.71,76
We found 32 documents with statements of the incubation period for CHIKV that provided two original estimates, 12 sourced estimates, and 21 unsourced estimates. (Three documents provided two estimates each.) Both of the original estimates were from observational studies. Only five of the sourced estimates were from primary sources. Several of these came from the original report of CHIKV—a 1955 publication of the 1952–1953 epidemic in Zanzibar.77 The author of this report estimated the incubation period of CHIKV from several hospital patients who had a single contact with an infectious village. Most estimates are consistent with an incubation period of 2–12 days (Table 1).
We extracted a total of 21 observations of the incubation period from eight observational studies. We estimated a median incubation period of 3.0 days (95% CI: 0.5–3.1) with a dispersion of 1.04 (95% CI: 1.04–1.08).
The study with the most observations (13) reported demographic and epidemiologic data on travelers diagnosed with chikungunya while visiting Italy between July and September 2006. Because the exact date and time of the infectious mosquito bite could not be identified, the incubation period estimates for these travelers ranged from < 1 day to a maximum of 31 days.16 We also found two articles reporting vertical transmission, but these cases were excluded from the analysis.78,79 One of these reports involved 19 infants born to 61 infected mothers on La Reunion Island over a 22-month period.78 The median time from delivery to when the infants presented symptoms was 4 days with a range of 3–7 days. The other report of vertical transmission was also from La Reunion Island, included six infants, and estimated the incubation period range to be 3–5 days.79
Dengue virus.
The DENV is a mosquito-borne virus with four distinct serotypes that cause a flu-like illness, fever, and sometimes a more severe complication called dengue hemorrhagic fever. The DENV is endemic to over 100 countries, and, according to the World Health Organization (WHO), ∼2/5 of the world's population are now at risk.1 The DENV is transmitted by Ae. mosquitoes, primarily by Ae. aegypti and secondarily by Ae. albopictus. Both mosquito species have geographically spread in recent years—Ae. aegypti particularly in urban areas and Ae. albopictus particularly in temperate regions (e.g., the southern portion of the United States).76 Several vaccine candidates are currently under study.1,80
We found 100 papers that provided a total of 135 incubation period estimates for DENV. (Several papers provided multiple and/or a combination of sourced, unsourced, and original estimates.) Of these estimates, 13 were based on original data and 56 were sourced. The most commonly referenced primary source was a book chapter by military officer J. F. Siler and others, which reported an experiment performed on 47 patients in 1924 in the Philippines.32 More recent case definitions included serologic confirmation, but those extracted from studies published in the late 19th and early 20th centuries were based primarily on clinical symptoms and history of exposure. Most estimates are consistent with an incubation period of 3–14 days (Table 1).
We extracted a total of 169 individual-level observations of the incubation period, including 128 observations from experimental studies. Based on these 169 observations, we estimate the median incubation period for DENV to be 5.6 days (95% CI: 5.3–6.0) with a dispersion of 1.41 days (95% CI: 1.34–1.50) (Table 3). Twenty-five percent of cases developed symptoms by 4.5 days following infection (95% CI: 4.1–4.9), and 75% developed symptoms by 7.1 days (95% CI: 6.7–7.6). There were insufficient data to confidently estimate the 5th or 95th percentiles. Restricting the analysis to the 124 individual-level observations from mosquito-transmitted infections, including 85 observations from experimental studies, we estimated a median incubation period of 5.3 days (95% CI: 5.0–5.7) with a dispersion of 1.37 (95% CI: 1.27–1.52). These estimates agree with a recent paper in which the authors completed a systematic review of the intrinsic incubation period of DENV.81 Based on 204 observations from 35 studies, they estimated a mean incubation period of 5.9 days and 95% of cases developed symptoms between 3.4 and 10 days.
Japanese encephalitis virus.
The JEV is a mosquito-borne virus, amplified in pigs and birds, and found throughout Asia and parts of Australia.82–84 The JEV is reported to be responsible for 50,000 cases and 10,000 deaths annually,85,86 but up-to-date estimates are lacking, possibly caused by the absence of encephalitis surveillance systems in several affected Asian countries.87 Before the introduction of vaccination, nearly all children in endemic countries were infected, but infections were usually asymptomatic.88
We found 35 documents with statements of the incubation period for JEV that provided two original estimates, nine sourced estimates, and 25 unsourced estimates. Of the two original estimates, both derived from observational studies. One of the sourced estimates was a report of an elderly Swedish tourist who acquired Japanese encephalitis while traveling in Java and Bali.44 The patient developed encephalitis after a reported incubation period of up to 17 days, but it is plausible that infection could have occurred at any point during the 3-week visit, resulting in an incubation period between four and 26 days. In addition to this case, we found only five other documents containing data with ascertainable incubation periods ranging from 0 to 23 days. All were case reports of infected tourists.
We only found data on six individual ascertainable incubation periods. Using these limited data, we estimate that JEV has a median incubation period of 8.4 days (95% CI: 5.1–9.4). This is similar to the central tendency of 7 days reported in the literature and falls within the 5–15 days incubation period often referenced (Table 1).9
Rift Valley fever virus.
The Rift Valley fever is largely a disease of livestock. However, it bears medical importance, as RVFV spreads to humans during livestock outbreaks by direct contact and mosquito transmission.89 For example, there were ∼200,000 human cases during a livestock outbreak in Egypt in 1977–78.90 Despite the disease's deadly effect on livestock, it typically presents as a mild, influenza-like illness in humans. However, life-threatening complications, including hemorrhagic fever and encephalitis, develop in about 1% of cases.91 This is a non-trivial number in large outbreaks—during the 1977–78 Egyptian outbreak, there were an estimated 600 deaths.90 The RVFV has been confined to the African continent until recently when outbreaks were confirmed in both Saudi Arabia and Yemen.92
We found 16 documents with statements of the incubation period for RVFV that provided four original estimates, three sourced estimates, and 10 unsourced estimates. Of the four original estimates, all derived from observational studies. The observational study with the most data came from a 1951 report of the incubation period in several South African veterinarians and farmhands who handled the organs of a bull infected with RVFV.10 Estimates are consistent with an incubation period of 3–6 days (Table 1).
Based on 23 observations from one experimental and seven observational studies, we estimate the median incubation period for RVFV to be 4.0 days (95% CI: 3.4–4.9), with a dispersion of 1.50 (95% CI: 1.22–1.82). Twenty-five percent of cases developed symptoms by 3.1 days (95% CI: 2.7–3.7) and 75% by 5.3 days (95% CI: 4.0–7.1) after infection. There were insufficient data to confidently estimate the 5th or 95th percentiles. However, it is important to note that at least 17 of these 18 observations were not transmitted by mosquitoes. Most were laboratory infections or infections following the butchering or postmortem examination of deceased, infected livestock. The sole experimental incubation period data came from the first published paper on the disease. Veterinary investigators of an outbreak among sheep on a farm in the Kenyan Rift Valley inoculated a hospitalized adult male with the virus and reported an incubation period of about 3 days.46,91
West Nile virus.
The geographic distribution of WNV is nearly global after rapidly expanding over the past two decades to include the United States, Canada, Mexico, the Caribbean, and portions of South America.86,93 The first documented outbreak in the Western Hemisphere was in New York City in 1999 and incurred 59 hospitalized cases and seven deaths.53–95 In endemic regions, WNV typically manifests as a mild or dengue-like illness,96 but among more recently exposed populations, neuroinvasive symptoms (e.g., meningitis, meningoencephalitis, and encephalitis) may be more common.52,97 For instance, in Queens, during the 1999 New York City outbreak, there was one meningoencephalitis case estimated for every 140 infections.98
We found 76 documents with statements of the incubation period for WNV that provided five original estimates, 32 sourced estimates, and 51 unsourced estimates. Of the five original estimates, one derived from an experimental study and four derived from observational studies. The experimental study, published in 1952, involved inoculating advance-stage cancer patients with WNV.55 A related experiment by the same authors contributed individual-level data and is discussed below. One of the observational studies with the most data was reported by researchers in Israel in 1952. During an outbreak of WNV in the Kibbutz Mayan Tsevi, the group of researchers were able to estimate the incubation period from individuals' movements into and out of the kibbutz.54 Incubation period estimates for WNV are consistent with an interval of 3–14 days (Table 1).
In 2002, four organ transplant patients in the Southeastern region of the United States were infected with WNV through a common donor99; this was the first documented instance of WNV transmission through organ transplantation. After incubation periods ranging from 6 to 18 days, three of the four transplant recipients developed encephalitis—two required mechanical ventilation support and one died. Our review returned individual-level data from which incubation periods could be estimated for five transplant patients and one blood transfusion patient. For these six patients, the median incubation period was 10.8 days with an interquartile range of 8.4–14.2 days.
Based on 18 observations from one experimental and four observational studies, we estimate the median incubation period for WNV to be 2.6 days (95% CI: 1.6–3.5), with a dispersion of 1.82 (95% CI: 1.27–2.67). Twenty-five percent of cases develop symptoms by 1.7 days (95% CI: 0.9–2.5) and 75% by 3.8 days (95% CI: 2.5–5.6) after infection. Five percent of cases develop symptoms by 1.0 day (95% CI: 0.4–1.9) and 95% by 7.0 days (95% CI: 3.6–12.4) after infection.
Of these 18 observations, eight were likely mosquito transmitted. We estimate the median incubation period for mosquito-transmitted WNV to be 2.9 days (95% CI: 0.5–3.1), with a dispersion of 1.04 (95% CI: 1.04–1.29).
Yellow fever virus.
Mild manifestation of YFV is clinically indistinguishable from several of the previous viruses (e.g., RVFV, DENV).86 Severe cases, however, develop jaundice (for which YFV is named), increased vomiting and, in 15–25% of cases, hemorrhage.86,100 Persistent underreporting of yellow fever cases challenges estimates of the human toll,101 however the WHO estimates 200,000 cases and 30,000 deaths annually, most of which occur in Africa and Central and South America. Vaccination is the central prevention strategy with campaigns administered effectively since the 1940s86; in 2008, routine immunization provided coverage to 91% of eligible adults and children in the Americas and 43% in Africa. Eight African countries that conducted targeted campaigns were able to reach over 90% of the eligible population.102
We found 56 documents with statements of the incubation period for YFV that provided 8 original estimates, 17 sourced estimates, and 39 unsourced estimates. Of the 8 original estimates, one derived from an experimental study and seven derived from observational studies. Most estimates are consistent with an incubation period of 3–6 days (Table 1).
Based on 91 observations from one experimental and 13 observational studies, we estimate the median incubation period for YFV to be 4.4 days (95% CI: 4.0–5.0) with a dispersion of 1.66 (95% CI: 1.48–1.82). Twenty-five percent of cases develop symptoms by 3.2 days (95% CI: 2.8–3.5) and 75% by 6.3 days (95% CI: 5.3–7.3) after infection. Five percent of yellow fever cases developed symptoms by 1.9 days (95% CI: 1.7–2.3), and 95% of cases developed symptoms by 10.3 days (95% CI: 7.9–12.8) following infection. After excluding 11 observations that were not transmitted by mosquitoes, incubation period estimates remained nearly unchanged with a median of 4.4 days (95% CI: 3.9–5.0), dispersion of 1.67 (95% CI: 1.47–1.84), 25th percentile of 3.1 days (95% CI: 2.8–3.5), and 75th percentile of 6.2 days (95% CI: 5.1–7.4).
We also found four papers reporting cases of yellow fever following vaccination, but these cases were excluded from the analysis103–106; three of the four reports were from the July 14, 2001 issue of the Lancet and detailed serious adverse events from vaccination with the 17D and 17DD YFV substrains. The reported incubation period for these cases ranged from 2 to 4 days.
Discussion
Given the global spread of mosquito-borne viruses in recent years, knowledge of their incubation periods assumes increasing importance in efforts to identify and control epidemics. However, statements of the incubation periods are typically a weak point in the literature. In our review, we found that 57% of the 375 estimates were uncited, and of those that were cited, over half cited an unsourced estimate. In total, the 375 incubation period estimates could be traced back to a mere 32 original data sources. To address this gap, we systematically reviewed the literature and characterized the incubation periods of CHIKV, DENV, JEV, RVFV, WNV, and YFV using individual-level observations from as far back as 1898.
There are several sources of uncertainty that limit our estimates. Uncertainty in the timing of infection and illness motivated our use of doubly interval-censored data, which accounts for uncertainty in both the period of infection and period of illness onset.8 Uncertainty in the time of infection may be further compounded as different studies may have slightly different definitions of the start of infection. To limit this source of variability, we defined onset as the time that any flu-like symptom (e.g., fever, chills) was reported. We believe our approach is conservative.
There is little published data on the incubation periods of JEV, RVFV, and CHIKV. For example, our estimates for the incubation period of JEV were only based on six studies. This lack of data is reflected in the wide confidence intervals for the median incubation period estimates and the inability to estimate the 5th and 95th percentiles of the incubation period for these viruses. Additional observational studies with details on the exposure period and illness onset are important to characterize these incubation periods with more precision. In addition, the majority of infections are not published. If these unpublished cases systematically differ from published cases, then our results may not be representative of all the infections.
Incubation periods may differ by mode of infection (e.g., mosquito, injection, aerosol, transfusion/transplant, and mother-to-child), immune status, and viral strain. We attempted to account for this effect heterogeneity where possible by estimating incubation periods separately for mosquito- and non-mosquito-transmitted infections, and separately for transplant and transfusion infections in the case of WNV. We found that incubation periods were similar between mosquito- and non-mosquito, non-transfusion-transmitted infections for DENV, WNV, and YFV. (CHIKV, RVFV, and JEV did not have enough data to make this comparison.) Incubation periods for West Nile transplant/transfusion-acquired infections were longer than for mosquito-acquired infections. However, more observational data is needed to precisely characterize the incubation periods for these subgroups and others—particularly in vulnerable subpopulations such as infants.
Despite limitations stemming from a lack of observational data, a key strength of this analysis is its compilation and use of available published data to fill a gap in the understanding of several mosquito-borne viruses and the diseases they cause. Climate change will continue to facilitate the spread of mosquito habitats. Indeed, the Asian tiger mosquito—which is capable of carrying CHIKV, DENV, WNV, and YFV74,76,107,108 is now found as far north as the Netherlands in Europe and Minnesota in the United States.109,110 Identification and control of mosquito-borne virus outbreaks will become increasingly important as mosquito habitats expand to previously unexposed populations. Our characterizations of these incubation periods provide a level of detail useful for outbreak prediction, management, and control efforts.
Footnotes
Authors' addresses: Kara E. Rudolph, Justin Lessler, Brittany Kmush, and Derek A. T. Cummings, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, E-mails: krudolph@jhsph.edu, jlessler@jhsph.edu, bkmush@jhsph.edu, and dcumming@jhsph.edu. Rachael M. Moloney, Center for Medical Technology Policy, Baltimore, MD, E-mail: rachael.moloney@gmail.com.
References
- 1.World Health Organization Dengue and severe dengue. 2012. www.who.int/mediacentre/factsheets/fs117/en Available at. Accessed November 7, 2012.
- 2.Centers for Disease Control and Prevention West Nile virus: final annual maps and data for 1999–2012. 2013. www.cdc.gov/westnile/statsMaps/finalMapsData/index.html Available at. Accessed June 10, 2013.
- 3.Lessler J, Brookmeyer R, Reich NG, Nelson KE, Cummings DA, Perl TM. Identifying the probable timing and setting of respiratory virus infections. Infect Control Hosp Epidemiol. 2010;31:809–815. doi: 10.1086/655023. [DOI] [PubMed] [Google Scholar]
- 4.Lessler J, Brookmeyer R, Perl TM. An evaluation of classification rules based on date of symptom onset to identify health-care associated infections. Am J Epidemiol. 2007;166:1220–1229. doi: 10.1093/aje/kwm188. [DOI] [PubMed] [Google Scholar]
- 5.Fraser C, Riley S, Anderson RM, Ferguson NM. Factors that make an infectious disease outbreak controllable. Proc Natl Acad Sci USA. 2004;101:6146–6151. doi: 10.1073/pnas.0307506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lessler J, Reich NG, Brookmeyer R, Perl TM, Nelson KE, Cummings DA. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infect Dis. 2009;9:291–300. doi: 10.1016/S1473-3099(09)70069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reich NG, Perl TM, Cummings DA, Lessler J. Visualizing clinical evidence: citation networks for the incubation periods of respiratory viral infections. PLoS ONE. 2011;6:e19496. doi: 10.1371/journal.pone.0019496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reich NG, Lessler J, Cummings DA, Brookmeyer R. Estimating incubation period distributions with coarse data. Stat Med. 2009;28:2769–2784. doi: 10.1002/sim.3659. [DOI] [PubMed] [Google Scholar]
- 9.Perez-Pina F, Merikangas UR. Japanese B encephalitis in an American soldier returning from Korea. N Engl J Med. 1953;249:531–532. doi: 10.1056/NEJM195309242491305. [DOI] [PubMed] [Google Scholar]
- 10.Mundel B, Gear J. Rift valley fever. I. The occurrence of human cases in Johannesburg. S Afr Med J. 1951;25:797–800. [PubMed] [Google Scholar]
- 11.Hoogstraal H, Meegan JM, Khalil GM, Adham FK. The Rift Valley fever epizootic in Egypt 1977–78. 2. Ecological and entomological studies. Trans R Soc Trop Med Hyg. 1979;73:624–629. doi: 10.1016/0035-9203(79)90005-1. [DOI] [PubMed] [Google Scholar]
- 12.Kokernot RH, McIntosh BM. Isolation of West Nile virus from a naturally infected human being and from a bird, Sylvietta rufescens (Vieillot) S Afr Med J. 1959;33:987–989. [PubMed] [Google Scholar]
- 13.ICDDR First identified outbreak of chikungunya in Bangladesh, 2008. Health and Science Bulletin. 2009;7:1–6. [Google Scholar]
- 14.Centers for Disease Control and Prevention Chikungunya fever diagnosed among international travelers—United States, 2005–2006. MMWR Morb Mortal Wkly Rep. 2006;55:1040–1042. [PubMed] [Google Scholar]
- 15.Parola P, de Lamballerie X, Jourdan J, Rovery C, Vaillant V, Minodier P, Brouqui P, Flahault A, Raoult D, Charrel R. Novel chikungunya virus variant in travelers returning from Indian Ocean islands. Emerg Infect Dis. 2006;12:1493–1499. doi: 10.3201/eid1210.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beltrame A, Angheben A, Bisoffi Z, Monteiro G, Marocco S, Calleri G, Lipani F, Gobbi F, Canta F, Castelli F, Gulletta M, Bigoni S, Del Punta V, Iacovazzi T, Romi R, Nicoletti L, Ciufolini MG, Rorato G, Negri C, Viale P. Imported chikungunya infection, Italy. Emerg Infect Dis. 2007;13:1264–1266. doi: 10.3201/eid1308.070161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Receveur M, Ezzedine K, Pistone T, Malvy D. Chikungunya infection in a French traveller returning from the Maldives, October, 2009. Euro Surveill. 2010;15:19494. doi: 10.2807/ese.15.08.19494-en. [DOI] [PubMed] [Google Scholar]
- 18.Tang JW. Chikungunya–China (Hong Kong) ex Mauritius: confirmed. ProMED-mail. 2006 www.promedmail.org/?archiveid=20060402.0989 Available at. Accessed November 30, 2012. [Google Scholar]
- 19.Soon YY, Junaidi I, Kumarasamy V, Chem YK, Juliana R, Chua KB. Chikungunya virus of Central/East African genotype detected in Malaysia. Med J Malaysia. 2007;62:214–217. [PubMed] [Google Scholar]
- 20.Volpe A, Caramaschi P, Angheben A, Marchetta A, Monteiro G, Banbara LM, Bisoffi Z. Chikungunya outbreak–remember the arthropathy. Rheumatology (Oxford) 2006;45:1449–1450. doi: 10.1093/rheumatology/kel275. [DOI] [PubMed] [Google Scholar]
- 21.Ashburn PM, Caraig CF. Experimental investigations regarding the etiology of dengue fever. J Infect Dis. 1907;4:440–475. doi: 10.1086/383418. [DOI] [PubMed] [Google Scholar]
- 22.Bancroft TL. On the etiology of dengue fever. Aust Med Gaz. 1906;25:17–18. [Google Scholar]
- 23.Cleland JB, Bradley B, Macdonald W. Further experiments in the etiology of dengue fever. J Hyg (Lond) 1919;18:217–254. doi: 10.1017/s0022172400007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeo PS, Pinheiro L, Tong P, Lim PL, Sitoh YY. Hippocampal involvement in dengue fever. Singapore Med J. 2005;46:647–650. [PubMed] [Google Scholar]
- 25.Cleland JB, Bradley B. Dengue fever in Australia: its history and clinical course, its experimental transmission by Stegomyia fasciata, and the results of inoculation and other experiments. J Hyg (Lond) 1918;16:317–418. doi: 10.1017/s0022172400006690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito M, Yamada K, Takasaki T, Pandey B, Noerome R, Tajima S, Morita K, Kurane I. Phylogenetic analysis of dengue viruses isolated from imported dengue patients: possible aid for determining the countries where infections occurred. J Travel Med. 2007;14:233–244. doi: 10.1111/j.1708-8305.2007.00130.x. [DOI] [PubMed] [Google Scholar]
- 27.Senanayake S. Dengue fever and dengue hemorrhagic fever–a diagnostic challenge. Aust Fam Physician. 2006;35:609–612. [PubMed] [Google Scholar]
- 28.Courtney M, Shetty AK. Imported dengue fever: an important reemerging disease. Pediatr Emerg Care. 2009;25:769–772. doi: 10.1097/PEC.0b013e3181bec8c7. [DOI] [PubMed] [Google Scholar]
- 29.Hanna JN, Ritchie SA, Hills SL, Pyke AT, Montgomery BL, Richards AR, Piispanan JP. Dengue in North Queensland, 2002. Commun Dis Intell Q Rep. 2003;27:384–389. [PubMed] [Google Scholar]
- 30.Hill DR. Evaluation of the returned traveler. Yale J Biol Med. 1992;65:343–356. [PMC free article] [PubMed] [Google Scholar]
- 31.Graham H. The dengue: a study of its pathology and mode of propagation. J Trop Med Hyg. 1903;6:209–214. [Google Scholar]
- 32.Siler JF, Hall MW, Kitchens AP. Dengue: its history, epidemiology, mechanism of transmission, etiology, clinical manifestations, immunity and prevention. Philipp J Sci. 1926;29:476. [Google Scholar]
- 33.Simmons JS, St John JH, Reynolds FHK. Experimental studies of dengue. Philipp J Sci. 1931;44:1–247. [Google Scholar]
- 34.Schule PA. Dengue fever: transmission by Aedes aegypti. Am J Trop Med Hyg. 1928;8:203–213. [Google Scholar]
- 35.Chandler AC, Rice L. Observations on the etiology of dengue fever. Am J Trop Med Hyg. 1923;3:223–262. [Google Scholar]
- 36.Hare FE. The 1897 epidemic of dengue in N. Queensland. Australas Med Gaz. 1898;17:98–107. [Google Scholar]
- 37.Koizumi T, Yamaguchi K, Tonomura K. A study of dengue fever. Chin Med J. 1918;33:355–357. [Google Scholar]
- 38.Agramonte A. Some clinical notes upon a recent epidemic of dengue fever. New York Med J. 1906;84:231–233. [Google Scholar]
- 39.Vassal JJ, Brochet A. Dengue in indo-China. Philipp J Sci. 1909;4:21–36. [Google Scholar]
- 40.Buhl MR, Black FT, Andersen PL, Laursen A. Fatal Japanese encephalitis in a Danish tourist visiting Bali for 12 days. Scand J Infect Dis. 1996;28:189. doi: 10.3109/00365549609049074. [DOI] [PubMed] [Google Scholar]
- 41.Caramello P, Canta F, Balbiano R, Lipani F, Ariaudo S, De Agostini M, Calleri G, Boglione L, Di Caro A. A case of imported JE acquired during short travel in Vietnam. Are current recommendations about vaccination broader? J Travel Med. 2007;14:346–348. doi: 10.1111/j.1708-8305.2007.00140.x. [DOI] [PubMed] [Google Scholar]
- 42.Lehtinen VA, Huhtamo E, Siikamaki H, Vapalahti O. Japanese encephalitis in a Finnish traveler on a two-week holiday in Thailand. J Clin Virol. 2008;43:93–95. doi: 10.1016/j.jcv.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Macdonald WB, Tink AR, Ouvrier RA, Menser MA, de Silva LM, Maim H, Hawkes RA. Japanese encephalitis after a two-week holiday in Bali. Med J Aust. 1989;150:334–336. doi: 10.5694/j.1326-5377.1989.tb136498.x. 339. [DOI] [PubMed] [Google Scholar]
- 44.Ostlund MR, Kan B, Karlsson M, Vene S. Japanese encephalitis in a Swedish tourist after travelling to Java and Bali. Scand J Infect Dis. 2004;36:512–513. doi: 10.1080/00365540410020640. [DOI] [PubMed] [Google Scholar]
- 45.Wittesjo B, Eitrem R, Niklasson B, Vene S, Mangiafico JA. Japanese encephalitis after a 10-day holiday in Bali. Lancet. 1995;345:856–857. doi: 10.1016/s0140-6736(95)92990-8. [DOI] [PubMed] [Google Scholar]
- 46.Daubney R, Hudson JR, Garnham PC. Enzootic hepatitis or Rift Valley fever. An undescribed virus disease of sheep, cattle, and man from East Africa. J Pathol Bacteriol. 1931;34:545–579. [Google Scholar]
- 47.Findlay GM. Rift Valley fever or enzootic hepatitis. Trans R Soc Trop Med Hyg. 1932;25:229–265. [Google Scholar]
- 48.Francis T, Magill TP. Rift Valley fever: a report of three cases of laboratory infection and the experimental transmission of the disease to ferrets. J Exp Med. 1935;62:433–448. doi: 10.1084/jem.62.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kitchen SF. Laboratory infections with the virus of Rift Valley fever. Am J Trop Med Hyg. 1934;14:547–564. [Google Scholar]
- 50.Sabin AB, Blumberg RW. Human infection with Rift Valley fever virus and immunity twelve years after single attack. Proc Soc Exp Biol Med. 1947;64:385–389. doi: 10.3181/00379727-64-15803. [DOI] [PubMed] [Google Scholar]
- 51.Smithburn KC, Mahaffy AF, Haddow AJ, Kitchen SF, Smith JF. Rift Valley fever; accidental infections among laboratory workers. J Immunol. 1949;62:213–227. [PubMed] [Google Scholar]
- 52.Campbell GL, Marfin AA, Lanciotti RS, Gubler DJ. West Nile virus. Lancet Infect Dis. 2002;2:519–529. doi: 10.1016/s1473-3099(02)00368-7. [DOI] [PubMed] [Google Scholar]
- 53.Nash D, Mostashari F, Fine A, Miller J, O'Leary D, Murray K, Huang A, Rosenberg A, Greenberg A, Sherman M, Wong S, Layton M. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344:1807–1814. doi: 10.1056/NEJM200106143442401. [DOI] [PubMed] [Google Scholar]
- 54.Olejnik E. Infectious adenitis transmitted by Culex molestus. Bull Res Counc Isr. 1952;2:210–211. [Google Scholar]
- 55.Southam CM, Moore AE. Clinical studies of viruses as antineoplastic agents with particular reference to Egypt 101 virus. Cancer. 1952;5:1025–1034. doi: 10.1002/1097-0142(195209)5:5<1025::aid-cncr2820050518>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 56.Hindle E. The transmission of yellow fever. Lancet. 1930;219:835–842. [Google Scholar]
- 57.Colebunders R, Mariage JL, Coche JC, Pirenne B, Kempinaire S, Hantson P, Van Gompel A, Niedrig M, Van Esbroeck M, Bailey R, Drosten C, Schmitz H. A Belgian traveler who acquired yellow fever in the Gambia. Clin Infect Dis. 2002;35:e113–e116. doi: 10.1086/344180. [DOI] [PubMed] [Google Scholar]
- 58.Smith CE, Gibson ME. Yellow fever in South Wales, 1865. Med Hist. 1986;30:322–340. doi: 10.1017/s0025727300045737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McFarland JM, Baddour LM, Nelson JE, Elkins SK, Craven RB, Cropp BC, Chang GJ, Grindstaff AD, Criag AS, Smith RJ. Imported yellow fever in a United States citizen. Clin Infect Dis. 1997;25:1143–1147. doi: 10.1086/516111. [DOI] [PubMed] [Google Scholar]
- 60.Centers for Disease Control and Prevention Fatal yellow fever in a traveler returning from Venezuela, 1999. MMWR Morb Mortal Wkly Rep. 2000;49:303–305. [PubMed] [Google Scholar]
- 61.Low GC, Fairley NH. Laboratory and hospital infections with yellow fever in England. BMJ. 1931;1:125–128. doi: 10.1136/bmj.1.3655.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carter HR. A note on the spread of yellow fever in houses. Med Record. 1901;59:933–937. [Google Scholar]
- 63.Reed W. Recent researches concerning the etiology, propagation, and prevention of yellow fever, by the United States Army Commission. J Hyg (Lond) 1902;2:101–119. doi: 10.1017/s0022172400001856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berry GP, Kitchen SF. Yellow fever accidentally contracted in the laboratory: a study of seven cases. Am J Trop Med Hyg. 1931;11:365–434. [Google Scholar]
- 65.Bugher JC, Gast-Galvis A. The efficacy of vaccination in the prevention of yellow fever in Colombia. Am J Hyg. 1944;39:58–66. [Google Scholar]
- 66.Dudley SF. Yellow fever as seen by the medican officers of the Royal Navy in the 19th century. J R Nav Med Serv. 1933;19:151–165. [Google Scholar]
- 67.World Health Organization Yellow fever, 1998–1999. Wkly Epidemiol Rec. 2000;75:322–328. [PubMed] [Google Scholar]
- 68.Centers for Disease Control and Prevention Fatal yellow fever in a traveler returning from Amazonas, Brazil, 2002. MMWR Morb Mortal Wkly Rep. 2002;51:324–325. [PubMed] [Google Scholar]
- 69.Nolla-Salas J, Saballs-Radresa J, Bada JL. Imported yellow fever in vaccinated tourist. Lancet. 1989;2:1275. doi: 10.1016/s0140-6736(89)91877-1. [DOI] [PubMed] [Google Scholar]
- 70.Griffin DE. Alphaviruses. In: Knipe DM, Howely PM, editors. Field's Virology. Volume I. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 1023–1054. [Google Scholar]
- 71.World Health Organization Chikungunya. 2008. www.who.int/mediacentre/factsheets/fs327/en/ Available at. Accessed November 16, 2012.
- 72.Lo Presti A, Ciccozzi M, Cella E, Lai A, Simonetti FR, Galli M, Zehender G, Rezza G. Origin, evolution, and phylogeography of recent epidemic CHIKV strains. Infect Genet Evol. 2012;12:392–398. doi: 10.1016/j.meegid.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 73.Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3:e201. doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schwartz O, Albert ML. Biology and pathogenesis of chikungunya virus. Nat Rev Microbiol. 2010;8:491–500. doi: 10.1038/nrmicro2368. [DOI] [PubMed] [Google Scholar]
- 75.Watson R. Europe witnesses first local transmission of chikungunya fever in Italy. BMJ. 2007;335:532–533. doi: 10.1136/bmj.39332.708738.DB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lambrechts L, Scott TW, Gubler DJ. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl Trop Dis. 2010;4:e646. doi: 10.1371/journal.pntd.0000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robinson MC. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952–53. I: clinical features. Trans R Soc Trop Med Hyg. 1955;49:28–32. doi: 10.1016/0035-9203(55)90080-8. [DOI] [PubMed] [Google Scholar]
- 78.Gerardin P, Barau G, Michault A, Bintner M, Randrianaivo H, Choker G, Lenglet Y, Touret Y, Bouveret A, Grivard P, Le Roux K, Blanc S, Schuffenecker I, Courderc T, Arenzana-Seisdedos F, Lecuit M, Robillard PY. Multidisciplinary prospective study of mother-to-child chikungunya virus infections on the island of La Reunion. PLoS Med. 2008;5:e60. doi: 10.1371/journal.pmed.0050060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paquet C, Quatresous I, Solet JL, Sissoko D, Renault P, Pierre V, Cordel H, Lassalle C, Thiria J, Zeller H, Schuffnecker I. Chikungunya outbreak in Reunion: epidemiology and surveillance, 2005 to early January 2006. Euro Surveill. 2006;11:E060202–E060203. doi: 10.2807/esw.11.05.02891-en. [DOI] [PubMed] [Google Scholar]
- 80.Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, Moureau A, Saville M, Bouchenooghe A, Viviani S, Tornieporth NG, Lang J. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomized, controlled phase 2b trial. Lancet. 2012;380:1559–1567. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 81.Chan M, Johansson MA. The incubation periods of dengue viruses. PLoS ONE. 2012;7:e50972. doi: 10.1371/journal.pone.0050972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mackenzie JS. Emerging zoonotic encephalitis viruses: lessons from Southeast Asia and Oceania. J Neurovirol. 2005;11:434–440. doi: 10.1080/13550280591002487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Buescher EL, Scherer WF. Ecologic studies of Japanese encephalitis virus in Japan. IX. Epidemiologic correlations and conclusions. Am J Trop Med Hyg. 1959;8:719–722. doi: 10.4269/ajtmh.1959.8.719. [DOI] [PubMed] [Google Scholar]
- 84.Konno J, Endo K, Agatsuma H, Ishida N. Cyclic outbreaks of Japanese encephalitis among pigs and humans. Am J Epidemiol. 1966;84:292–300. doi: 10.1093/oxfordjournals.aje.a120643. [DOI] [PubMed] [Google Scholar]
- 85.Umenai T, Krzysko R, Bektimirov TA, Assaad FA. Japanese encephalitis: current worldwide status. Bull World Health Organ. 1985;63:625–631. [PMC free article] [PubMed] [Google Scholar]
- 86.Gubler DJ, Kuno G, Markoff L. Flaviviruses. In: Knipe DM, Howley PM, editors. Fields Virology. Volume I. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. p. 1185. [Google Scholar]
- 87.Touch S, Grundy J, Hills S, Rani M, Samnang C, Khalakdina A, Jacobson J. The rationale for integrated childhood meningoencephalitis surveillance: a case study from Cambodia. Bull World Health Organ. 2009;87:320–324. doi: 10.2471/BLT.08.052951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Solomon T, Vaughn DW. Pathogenesis and clinical features of Japanese encephalitis and West Nile virus infections. Curr Top Microbiol Immunol. 2002;267:171–194. doi: 10.1007/978-3-642-59403-8_9. [DOI] [PubMed] [Google Scholar]
- 89.Chevalier V, Pepin M, Plee L, Lancelot R. Rift Valley fever–a threat for Europe? Euro Surveill. 2010;15:19506. [PubMed] [Google Scholar]
- 90.Meegan JM. Rift Valley fever in Egypt: an overview of the epizootics in 1977 and 1978. Contr Epidem Biostatist. 1981;3:100–113. [Google Scholar]
- 91.Schmaljohn CS, Nichol ST. Bunyaviridae. In: Knipe DM, Howley PM, editors. Fields Virology. Volume II. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 1741–1779. [Google Scholar]
- 92.Shoemaker T, Boulianne C, Vincent MJ, Pezzanite L, Al-Qahtani MM, Al-Mazrou Y, Khan AS, Rollin PE, Swanepoel R, Ksiaszek TG, Nichol ST. Genetic analysis of viruses associated with emergence of Rift Valley fever in Saudi Arabia and Yemen, 2000–2001. Emerg Infect Dis. 2002;8:1415–1420. doi: 10.3201/eid0812.020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hayes EB, Gubler DJ. West Nile virus: epidemiology and clinical features of an emerging epidemic in the United States. Annu Rev Med. 2006;57:181–194. doi: 10.1146/annurev.med.57.121304.131418. [DOI] [PubMed] [Google Scholar]
- 94.Weiss D, Carr D, Kellachan J, Tan C, Phillips M, Bresnitz E, Layton M. Clinical findings of West Nile virus infection in hospitalized patients, New York and New Jersey, 2000. Emerg Infect Dis. 2001;7:654–658. doi: 10.3201/eid0704.010409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Centers for Disease Control and Prevention Update: West Nile virus encephalitis–New York, 1999. JAMA. 1999;282:1806–1807. [PubMed] [Google Scholar]
- 96.Hurlbut HS, Rizk F, Taylor RM, Work TH. A study of the ecology of West Nile virus in Egypt. Am J Trop Med Hyg. 1956;5:579–620. doi: 10.4269/ajtmh.1956.5.579. [DOI] [PubMed] [Google Scholar]
- 97.Tsai TF, Popovici F, Cernescu C, Campbell GL, Nedelcu NI. West Nile encephalitis epidemic in southeastern Romania. Lancet. 1998;352:767–771. doi: 10.1016/s0140-6736(98)03538-7. [DOI] [PubMed] [Google Scholar]
- 98.Mostashari F, Bunning ML, Kitsutani PT, Singer DA, Nash D, Cooper MJ, Katz N, Liljebjelke KA, Biggerstaff BJ, Fine AD, Layton MC, Mullin SM, Johnson AJ, Martin DA, Hayes EB, Campbell GL. Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet. 2001;358:261–264. doi: 10.1016/S0140-6736(01)05480-0. [DOI] [PubMed] [Google Scholar]
- 99.Centers of Disease Control and Prevention West Nile virus infection in organ donor and transplant recipients–Georgia and Florida, 2002. MMWR Morb Mortal Wkly Rep. 2002;51:790. [PubMed] [Google Scholar]
- 100.World Health Organization Yellow fever. 2012. www.who.int/mediacentre/factsheets/fs100/en Available at. Accessed November 30, 2012.
- 101.World Health Organization Yellow fever: trends over time. 2012. www.who.int/csr/disease/yellowfev/trends/en/index.html Available at. Accessed November 7, 2012.
- 102.World Health Organization Yellow Fever Initiative: providing an opportunity of a lifetime. 2010. www.who.int/csr/disease/yellowfev/YFIbrochure.pdf Available at. Accessed November 7, 2012.
- 103.Chan RC, Penney DJ, Little D, Carter IW, Roberts JA, Rawlinson WD. Hepatitis and death following vaccination with 17D-204 yellow fever vaccine. Lancet. 2001;358:121–122. doi: 10.1016/S0140-6736(01)05341-7. [DOI] [PubMed] [Google Scholar]
- 104.Martin M, Tsai TF, Cropp B, Chang GJ, Holmes DA, Tseng J, Shieh W, Zaki SR, Al-Anouri I, Cutrona AF, Ray G, Weld LH, Cetron MS. Fever and multisystem organ failure associated with 17D-204 yellow fever vaccination: a report of four cases. Lancet. 2001;358:98–104. doi: 10.1016/s0140-6736(01)05327-2. [DOI] [PubMed] [Google Scholar]
- 105.Van Langenberg E. Acute necrosis of the liver: an unusual case. Lancet. 1944;243:244–245. [Google Scholar]
- 106.Vasconcelos PF, Luna EJ, Galler R, Silva LJ, Barros VL, Monath TP, Rodigues SG, Laval C, Costa ZG, Vilela MF, Santos CL, Papaiordanou RM, Alves VA, Andrade LD, Sato HK, Rosa ES, Froguas GB, Lacava E, Almeida LM, Cruz AC, Rocco IM, Sanots RT, Oliva OF. Serious adverse events associated with yellow fever 17DD vaccine in Brazil: a report of two cases. Lancet. 2001;358:91–97. doi: 10.1016/s0140-6736(01)05326-0. [DOI] [PubMed] [Google Scholar]
- 107.Gubler DJ, Reiter P, Ebi KL, Yap W, Nasci R, Patz JA. Climate variability and change in the United States: potential impacts on vector- and rodent-borne diseases. Environ Health Perspect. 2001;109((Suppl 2)):223–233. doi: 10.1289/ehp.109-1240669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Holick J, Kyle A, Ferraro W, Delaney RR, Iwaseczko M. Discovery of Aedes albopictus infected with west Nile virus in southeastern Pennsylvania. J Am Mosq Control Assoc. 2002;18:131. [PubMed] [Google Scholar]
- 109.European Centre for Disease Prevention and Control Aedes albopictus: current known distribution, September 2012. 2012. ecdc.europa/eu/en/activities/diseaseprogrammes/emerging_and_vector_borne_diseases/PublishingImages/Aalbopictus-maps-distribution-september2012-high-res.jpeg Available at. Accessed November 30, 2012.
- 110.Centers for Disease Control and Prevention Distribution of Aedes albopictus in the United States, by county, 2000. 2005. www.cdc.gov/ncidod/dvbid/arbor/albopic_97_sm.htm Available at. Accessed November 30, 2012.