DNA methylation dynamics in neurogenesis (original) (raw)
Abstract
Neurogenesis is not limited to the embryonic stage, but continually proceeds in the adult brain throughout life. Epigenetic mechanisms, including DNA methylation, histone modification and noncoding RNA, play important roles in neurogenesis. For decades, DNA methylation was thought to be a stable modification, except for demethylation in the early embryo. In recent years, DNA methylation has proved to be dynamic during development. In this review, we summarize the latest understanding about DNA methylation dynamics in neurogenesis, including the roles of different methylation forms (5-methylcytosine, 5-hydroxymethylcytosine, 5-formylcytosine and 5-carboxylcytosine), as well as their ‘writers’, ‘readers’ and interactions with histone modifications.
Keywords: : DNA methylation, DNA methyltransferase, histone modification, neurogenesis, TET family protein
Neural stem and neural progenitor cells (NSCs, NPCs) generate neurons via neurogenesis [1], which was originally thought to occur only during embryonic development. It is now clear that neurogenesis is not restricted to the embryonic stage. In the adult subventricular zone (SVZ) and the subgranular zone (SGZ), neurons are robustly generated daily and integrated into existing circuits [2]. Extracellular signals, intrinsic regulators and epigenetic modifiers synergistically orchestrate the complex neurogenesis process in both the embryo and the adult stage.
Today, the widely accepted definition of ‘epigenetics’ is “any meiotically or mitotically heritable change in gene function that cannot be ascribed to changes in the primary DNA sequence.” [3]. People have realized that some dynamic flux in chromatin modifications is not necessarily heritable, but still results in changes of gene expression. As such, Bird proposed a modified definition of epigenetics: “the structural adaptation of chromosomal regions so as to register, signal or perpetuate altered activity states.” [4]. We prefer to adopt the latter definition as it avoids the constraints imposed by the requirement of heritability. Epigenetic mechanisms include DNA methylation, histone modification and noncoding regulatory RNAs (such as long noncoding RNA). DNA methylation was long viewed as a stable modification once established in the embryonic stage. The discovery of 5-hydroxymethylcytosine (5hmC) in mammals opened up a new avenue of DNA methylation research [5,6]. DNA methylation dynamics (methylation/demethylation) was established with the identification of more oxidized cytosine forms, including 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC), as well as enzymes including the ten-eleven translocation (TET) family and thymine DNA glycosylase (TDG) [7–11]. It has now become clear that cytosine methylation is highly dynamic [8,11–12]. However, DNA methylation dynamics during neurogenesis and brain development (neuroepigenetics) has been explored only in recent years.
In this review, we summarize the current understanding of DNA methylation dynamics in neurogenesis. We begin by describing the neurogenesis process in both the embryo and adult stages, followed by a brief overview of DNA methylation/demethylation and its writers. We then focus on the role(s) of DNA methylation at different stages of embryonic neurogenesis, as well as adult neurogenesis.
Fundamentals of neurogenesis
Embryonic neurogenesis
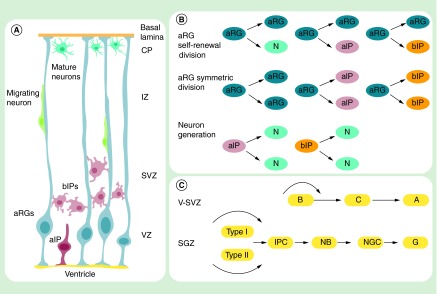
The process of neurogenesis is complex, within which many details remain unclear. Here, we provide a brief conclusion of current understanding of neurogenesis. We focus on the rodent models that have been extensively studied. In mammals, the nervous system develops from ectoderm. The neural plate fuses to form a neural tube. The lumen of this tube becomes the ventricular system, while neurogenesis proceeds within the walls of neural tube to form the CNS, including the brain and the spinal cord [13]. Before neurogenesis, the neural tube is composed of a single layer of neuroepithelial cells (NEs), which are primary NSCs. These cells first proliferate by symmetric divisions, enabling the expansion of the initial pool of cortical progenitors [14]. At mid-gestation, with the onset of neurogenesis, NEs start to undergo transformation to give rise to apical radial glial cells (aRGs). By E13/E14, aRGs constitute the majority of neural progenitors in most brain regions in the mouse (Figure 1A) [1,15]. aRGs are highly related to NEs but possess their own identity. Besides expressing a number of neuroepithelial markers, such as Nestin, aRGs also express some astroglial markers, including GLAST (glutamate-aspartate transporter) and BLBP [16]. Like NEs, aRGs are also bipolar cells, but have more elongated processes, with apical endfoot connected by adherens junctions lining the ventricle filled with cerebrospinal fluid (CSF), and the basal endfoot attached to the basal lamina [1,16]. The layer of aRGs forms the ventricular zone (VZ). aRGs function as neural stem cells and generate almost all cells in the CNS, including neurons, astrocytes, and oligodendrocytes [1]. Neurogenesis typically precedes gliogenesis [17]. During the neurogenesis stage, aRGs mainly generate neurons via the generation of apical intermediate progenitors (aIPs) and basal intermediate progenitors (bIPs) (Figure 1B) [1]. aIPs are bipolar cells, while bIPs are nonpolar cells. aIPs are located in the VZ, whereas bIPs form an additional proliferative zone basal to the VZ, called as SVZ. Both aIPs and bIPs can only undergo one round of symmetric consumptive division to generate neurons [14]. An aRG can undergo symmetric proliferative division to generate two aRGs, or asymmetric self-renewing division to generate one daughter aRG and one more differentiated cell, such as a neuron and bIP [1]. Nascent neurons migrate toward the basal direction along the basal process of aRGs, and gradually form the six-layer cortex structure in an inside-first, outside-last manner [18]. Neurogenesis peaks at E14, and recedes by E17 in late gestation, when the neurogenesis process of radial glial cells (RGs) is converted to astrocytogenesis and oligodendrocytogenesis, sequentially [19]. Astrocytogenesis peaks at postnatal day 2 (P2), and oligodendrocytogenesis at P14 [19]. In the embryonic stage, astrocytes and oligodendrocytes are generated from astrocyte intermediate progenitor cells and oligodendrocyte intermediate progenitor cells, respectively, which in turn are derived from aRGs [20]. Recent research shows that in mouse postnatal cortex, a major source of astrocytes is the local proliferation of differentiated astrocytes [21]. At the end of embryonic neurogenesis, most aRGs transform into astrocytes [16]. However, some aRGs transform into astroglial cells (type B1 cells) in the SVZ and work as neural stem cells in adult neurogenesis [16].
Figure 1. . Neurogenesis in the embryonic and adult stage.
(A) aRGs can produce aIPs and bIPs, which can be divided symmetrically to generate neurons. Newborn neurons migrate along the basal process of aRGs to form the cortical plate. (B) aRGs can undergo asymmetric self-renewing division to generate one daughter aRG and one more differentiated cell, such as a(n) neuron, aIP and bIP. aIP and bIP undergo one round of symmetric division to generate neurons. aRGs can also proliferate and generate aIP and bIP by symmetric divisions. (C) Cell lineage in the V-SVZ and SGZ during adult neurogenesis.
A: Type A cell; aIP: Apical intermediate progenitor; aRG: Apical radial glial cell; B: Type B cell; bIP: Basal intermediate progenitor; C: Type C cell; CP: Cortical plate; GC: Granular cell; IPC: Intermediate progenitor cell; IZ: Intermediate zone; N: Neuron; NB: Neuroblast; NGC: New granular cell; SGZ: Subgranular zone; SVZ: Subventricular zone; V-SVZ: Ventricular-subventricular zone; VZ: Ventricular zone.
The basic features of cortical neurogenesis appear to be well-conserved among mammals; however, a number of human-specific features have been identified as well [22]. For example, during human cortical neurogenesis, there is an extended period of initial amplification of NEs followed by a much protracted period of generation of neurons from progenitors, allowing the generation of a much larger number of neurons as compared with rodent [23].
Extracellular signals, intrinsic regulators and epigenetic modifiers synergistically orchestrate the complex neurogenesis process in both the embryo and adult stage. Before discussing DNA methylation dynamics in neurogenesis, which is the focus of this review, here (and also in the section ‘adult neurogenesis’) we briefly introduce cell-extrinsic factors, cell-intrinsic regulations and other forms of epigenetic regulations (i.e., histone modifications) in neurogenesis.
Proceeding in the apical-to-basal direction, extracellular signals may come from CSF, neighboring cells, blood vessels and meninges [1]. Signals that come from CSF include IGFs, FGFs, sonic hedgehog, BMPs, and Wnts [24]. IGFs, in particular IGF3, stimulate progenitor proliferation and influence brain size. FGF8 and FGF17 promote the development of anterior cortical areas, while FGF15 opposes this action, thus making up a complex triadic pattern of control [24].
Using pluripotent stem cells models, human-specific regulations in cortical neurogenesis has also been uncovered. In mouse model, Sox1 is the earliest marker gene expressed during neural commitment, while Pax6 is induced afterward [25]. This is contrast to the situation observed in the human pluripotent stem cell (PSC)-derived models, where Pax6 starts to express at much earlier time point and initiates the expression of Sox1 [25]. However, overall the mechanisms underlying the human-specific features of neurogenesis still need further investigation.
The important intrinsic regulation in neurogenesis requires orchestration between transcription factors and epigenetic regulation [26]. For example, REST, a complex with several corepressors, including CoREST, histone deacetylases and MeCP2, is important in the maintenance of the neural progenitor state and suppression of premature neuronal differentiation. REST binds to DNA-binding sequence, known as repressor element 1 (RE1), a specific site present in the promoters of some neuron-specific genes, such as Mash1, and silences the expression of those proneural genes via complex mechanisms, including histone deacetylation [27].
Adult neurogenesis
Under normal conditions, active adult mammalian neurogenesis is restricted to two regions: the SVZ that lines the walls of the lateral ventricle, and the dentate gyrus SGZ of the hippocampus (Figure 1C) [2]. The adult SVZ harbors radial glia-like cells (type B1 cells), which are the SVZ stem cells. B1 cells contact the ventricle with a thin cellular process that is interdigitated between ependymal cells. Recent research shows that B1 cells can exit in either a quiescent or activated state [28]. Proliferating B1 cells give rise to transient amplifying cells (C cells), which in turn generate neuroblasts (A cells). Through a tube formed by astrocytes, neuroblast cells form a chain, called the rostral migratory stream, and migrate toward the olfactory bulb, where they are converted to different subtypes of mature neurons [2]. Newborn olfactory bulb neurons are involved in cognitive functions, such as perceptual learning and olfactory memory [29].
In the SGZ, radial glia-like cells (type I cells) and nonradial precursor cells (type II cells) work as neural progenitors in the dentate gyrus. These cells produce intermediate progenitors, which in turn generate neuroblasts. Neuroblasts migrate into the inner granular cell layer and differentiate into dentate granular cells in the hippocampus [2]. Newborn hippocampal neurons are believed to adapt the brain to temporal events present in external space, including spatial learning and retention, pattern discrimination and clearance of memory traces [30]. Adult neurogenesis appears well-conserved among mammals. However, because of methodological challenges to study this process in human, it was not until recently that quantitative data on adult human neurogenesis became available [31]. There is substantial hippocampal neurogenesis in adult humans [32]; however, there is no detectable olfactory bulb neurogenesis but continuous neurons generation in the striatum, which makes human unique among mammals [31].
Like embryonic neurogenesis, adult neurogenesis also depends on the active integration of cell-autonomous and extrinsic cues. Cells in the ventricular-SVZ (V-SVZ) and SGZ receive extrinsic signals from neighboring cells like ependymal cells or neurons, and blood vessels. Type B1 cells in the V-SVZ can also accept signals from CSF. These signal molecules include Notch, sonic hedgehog, Wnts and BMPs, to name but a few. For example, in the adult SVZ, deletion of Rbpj, a downstream mediator of all Notch receptors, activates type B1 cells to differentiate into C cells, resulting in depletion of quiescent neural precursors and the loss of continuous neurogenesis [33]. Interestingly, after deletion of Notch1 or Rbpj in neural precursors, similar effects are found in the adult SGZ [34]. Intracellular regulators of adult neurogenesis also include transcription factors and epigenetic factors. For instance, inhibitors of DNA-binding genes are highly expressed in radial glia-like cells in both the adult SVZ and SGZ. High expression of inhibitors of DNA-binding genes, which encode dominant-negative antagonists of the proneural basic helix-loop-helix transcription factors, maintains the pluripotent states of the radial glia-like cells [35]. Dlx2 expression is required for normal neuronal differentiation in the adult SVZ. Mixed-lineage leukemia 1 (Mll1), a TrxG member that encodes an H3K4 methyltransferase, can directly target Dlx2 and add the active transcription marker H3K4 [36]. Further experiments supported a model in which Mll1 was required to resolve silenced bivalent Dlx2 loci in postnatal neural precursors to the actively transcribed state for the induction of neurogenesis [36]. On the other hand, environmental factors could also contribute to the regulation of neurogenesis. It has been shown that enriched environment and physical exercise could promote cell proliferation and new neuron survival [37,38], while a wide variety of stress stimuli that can lead to depression generally reduce the rate of adult SGZ neurogenesis [39], which are likely mediated by epigenetic mechanism(s).
DNA methylation/demethylation
For decades, methylation of the fifth position of cytosine (5-methylcytosine; 5mC) remained the only known direct functional epigenetic modification in mammalian DNA sequence, until the recognition of 5hmC in 2011 [5,6]. 5mC is found in most plants, animals and fungi, and has a profound impact on the regulation of development of gene expression [40]. In mammals, cytosine methylation occurs mostly in the context of palindromic CpG dinucleotides, while CpG islands located in gene promoters with high CpG density usually remain unmethylated [41]. Recent work has shown that non-CpG methylation is relatively abundant in oocytes, pluripotent embryonic stem cells (ESCs) and mature neurons, but the function of mammalian non-CpG methylation remains unclear [42,43]. In mammals, new DNA methylation patterns are established by de novo DNA methyltransferases (DNMT), DNMT3A and DNMT3B, which transfer a methyl group from the universal methyl donor S-adenosyl-l-methionine to the unmethylated cytosine in the genome [44,45]. Their activity can be regulated by a catalytically inactive family member, DNMT3-like protein (DNMT3L) [46]. During mitosis S phase, the CpG methylation pattern in the model strand is faithfully copied to the daughter strand by DNMT1 and its partner UHRF1 (also named NP95), which binds preferentially to the hemimethylated CpGs [47,48]. Among the roughly 28 million CpGs in the human somatic cell genome, 60–80% are methylated [49]. The primary function of 5mC is largely transcriptional repression of both genes and repetitive elements; however, this could depend on genomic context. The repression of DNA methylation is generally thought to occur via two mechanisms. First, methylation of CpG dinucleotides affects DNA structure, thus directly preventing the binding of methylation-sensitive transcriptional activators [50]. Second, and a more pervasive effect, 5mC can be recognized and interpreted by a series of ‘readers’, called as methyl-CpG-binding proteins, which can further recruit chromatin remodeling complexes. Among them, proteins containing methyl-binding domain (MBD) are well-studied, particularly MBD1 and MeCP2 [51]. The inheritability and suppressive effect of CpG methylation suggests a role for 5mC in long-term epigenetic regulation during diverse processes, such as stable silencing of gene expression, maintenance of genome stability and genomic imprinting [52].
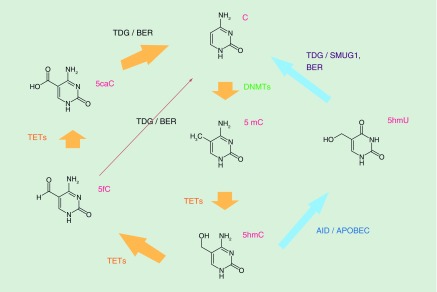
Although stably maintained in somatic cells, 5mC is found to undergo genome-wide demethylation during early embryo development [53]. In mouse sperm, roughly 80% of CpG dinucleotides are methylated, while oocytes possess a relatively low percentage of about 50%. The mouse germline undergoes two major waves of demethylation. The first wave starts in the zygote and ends in the blastocyst stage. The second wave occurs in the primordial germ cells (PGCs) between E6.5 and E13.5 [54]. Methylation erased primordial germ cells display <10% methylation. This genome-wide erasure of DNA methylation ensures that the genome is essentially devoid of epigenetic memory, which is important for setting up a pluripotent ground state. Surprisingly, the rapid demethylation of highly methylated sperm-derived DNA occurs even before the first S phase [53], which cannot be fully explained by DNMT1 activity suppression in the zygote. The active demethylation mechanism remained unknown until the discovery of a series of cytosine modification forms and related enzymes. In 2009, 5hmC was found in mouse genomes, and TET1 was identified as the enzyme that converts 5mC to 5hmC (Figure 2) [5,6]. Similar enzymatic activity is also seen in all three TET proteins (TET1–3) in mouse [7]. Subsequent studies revealed that TET proteins can further oxidize 5hmC to 5fC and 5caC (Figure 2) [9,10]. 5caC can be successively excised by TDG to generate an abasic site, which can then be repaired to a cytosine by the base excision repair (BER) pathway [9,55], completing the DNA methylation/demethylation cycle. It is noteworthy that other putative mechanisms have been put forward to explain the active erasure of 5mC (for review, see [54]). In the case of paternal pronucleus active demethylation, TET3 is responsible for the rapid increase of 5hmC, 5fC and 5caC [56–58]. However, TDG is not involved in removing these modifications, and it is thought that 5fC and 5caC are lost during DNA replication [57,59]. Thus, other mechanism besides the TDG/BER pathway may underlie this demethylation process.
Figure 2. . Dynamic DNA methylation/demethylation pathways.
The pathway indicated by orange arrows is most widely accepted nowadays, whereas the one indicated by blue arrows remains controversial. Notably, there are some additional putative demethylation mechanisms [54].
5caC: 5-carboxylcytosine; 5fC: 5-formylcytosine; 5hmc: 5-hydroxymethylcytosine; 5mc: 5-methylcytosine; BER: Base excision repair; TDG: Thymine DNA glycosylase.
Early embryo undergoes active DNA demethylation, resulting in the loss of nearly all modified cytosine (5mC and oxidized cytosine) by the 16-cell stage [53,60]. Approximately when the blastula implants into the uterus, there is an increase of activity in DNMT3A/B enzymes in the inner cell mass [41]. TET1 and TET2 are highly expressed at this stage, resulting in an increase of 5hmC, potentially fine-tuning methylation pattern. Intriguingly after this stage, TET1/2 expression levels decline steeply [41]. These enzyme expression changes can set up new epigenetic memories to define cellular identity and restrict lineage choices. Neurogenesis is a spatiotemporally orchestrated process that requires a precisely controlled gene regulatory program to generate diverse, functionally distinct populations of neurons and glia. Epigenetic modulation could contribute to this regulatory program.
DNA methylation writers & erasers in neurodevelopment & neurogenesis
As the primary writers of DNA methylation patterns, proper expression of DNMTs is particularly important for neurogenesis. DNMTs display spatial and temporal expression during neurogenesis (Figure 3). DNMT1 is substantially expressed in the ventricular neurogenic layer in embryonic mouse brain, consistent with its role in maintaining DNA methylation patterns throughout cell replication [61]. DNMT3B is robustly expressed in the VZ between E10.5 and E13.5, coinciding with vigorous embryonic neurogenesis. DNMT3B then becomes undetectable after E15.5 [62], while DNMT3A plays a more significant role in neurogenesis. DNMT3A is initially detected primarily in NPCs within the VZ and SVZ from E10.5 to E17.5, and continues to express in postnatal neurons across the brain, including the V-SVZ and hippocampal dentate gyrus, where adult neurogenesis occurs [62]. Inactivation of Dnmt1 and Dnmt3b by gene targeting in embryonic stem cells results in embryonic lethality [45,63]. Although Dnmt3a -/- mice develop to term and appear to be normal at birth, most homozygous mutant mice are runted and died at about 4 weeks of age due to multiple developmental defects, including growth impairment and rostral neural tube defects [45]. Conditional knockouts of Dnmts in mice were developed to study their role in the CNS. In the case of conditional Dnmt1 deletion in neuroblasts of E12 embryos, mutant embryos carrying 95% hypomethylated cells in the brain died immediately after birth due to respiratory distress. Although mosaic animals with 30% hypomethylated CNS cells were viable into adulthood, these mutant cells were eliminated quickly from the brain within 3 weeks of postnatal life, which also suggested that DNA hypomethylation disrupts the survival of CNS neurons [64]. In another mouse model, Dnmt1 was specifically deleted in retina from the onset of neurogenesis. Retinal progenitor cells continued to proliferate, whereas, there was an increased proportion of G1 phase cells. Although all major retinal neuronal cell types were produced, these cells showed defective differentiation and died rapidly in the postnatal retina [65]. Mice lacking functional Dnmt3a in the nervous system were born healthy, but showed various neural defects, including impairment of neuromuscular control on motor movement, and died prematurely [66]. These findings suggest that DNMTs play important roles in neurogenesis and neurodevelopment.
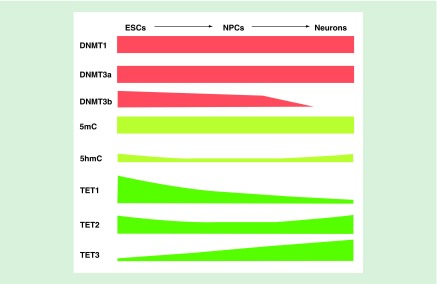
Figure 3. . Dynamics of 5-methylcytosine, 5-hydroxymethylcytosine, DNA methyltransferases and ten-eleven translocations during neurogenesis.
DNMT1 and DNMT3a are relatively stably expressed during neurogenesis process, while DNMT3b is highly expressed in ESCs and primarily expressed in NPCs in mammal central neural system. TET1 and TET2 are robustly expressed in ESCs, while TET3 is only marginally expressed. The expression level of TET1 decreased during neurogenesis process. The expression of TET2 maintains comparable level or decreased to some extent during ESCs differentiation into NPCs, which is showed by independent studies. TET3 and TET3 are robustly expressed during NPCs differentiation into neurons. Global level of 5mC remains unchanged, while methylation dynamic in some particular regions, such as low-methylated regions, is important for neurogenesis. 5hmC level decreases dramatically (˜80%) during ESCs differentiation into NPCs, while increases doubly in neurons in comparison to NPCs.
5hmc: 5-hydroxymethylcytosine; 5mc: 5-methylcytosine; DNMT: DNA methyltransferase; ESC: Embryonic stem cell; NPC: Neural progenitor cell.
As the key proteins involved in DNA demethylation, TET proteins (‘erasers’) also play crucial roles in neurogenesis (Figure 3). Tan et al. compared the expression levels of the TET gene family in mouse ESCs (mESCs) versus NPCs. Quantitative reverse transcription-PCR analysis revealed that Tet1 mRNA level, which was high in mESCs, decreased dramatically in NPCs. Tet2 mRNA levels were comparable between ESCs and NPCs, while Tet3 was upregulated in NPCs, but only marginally expressed in mESCs [67]. In other two independent studies, Tet1 was found to be downregulated and Tet3 was upregulated during mESC differentiation into NPCs, which were consistent with Tan et al. report. However, Tet2 was found to be downregulated. Different ESCs lines and differentiation protocols used in these studies might explain the inconsistency [68,69]. In situ hybridization in the mouse embryonic brain showed Tet3, Tet2 and Tet1 genes were expressed in an overall high-to-low order in the cortex. Tet3 was first expressed strongly in neuroepithelium in E11.5, consistent with the time of neurogenesis’ onset. During neuronal differentiation, there were noticeable increases of the levels of Tet3 and Tet2 in the cortical plate as well as the differentiated area of ganglionic eminence, compared with the ventricular area. At E15.5, cortical neurons also show higher expression of Tet3 compared with cortical NPCs. These data suggested the higher expression levels of Tet3 and Tet2 in differentiated neurons compared with NPCs, which might be involved in maintaining the proper progression of the differentiation process in neurogenesis [70].
Tet1 knockout and even Tet1/2 double knockout mice appear to be compatible with embryonic and postnatal development [71,72]. Although their brains showed normal morphology, adult Tet1 knockout (KO) mice had specific impairments in extinction learning and short-term memory [73,74]. Kass et al. overexpressed TET1 or a catalytically inactive mutant (TET1m) in the wild-type mouse hippocampus. Overexpression of both TET1 forms resulted in the upregulation of several neuronal memory-associated genes and impaired contextual fear memory, suggesting that TET1 plays an enzyme activity-independent role in memory formation [75]. Tet3 is important in early embryonic development. Half of the embryos that arose from Tet3 -/- oocytes, regardless of the sperm genotype, arrested around E11.5 and did not survive. The Tet3 -/ - mice that survived embryonic development died perinatally for unknown reasons [56]. Using Xenopus as a model, Xu et al. found that TET3 works as a transcriptional regulator in early eye development and neurodevelopment. Depleting endogenous TET3 protein greatly inhibited master neuronal development genes, such as Pax6, Rx and Six6 in the eye. The expression of neuronal genes, such as Ngn2 and Tubb2b, as well as the neural crest genes, Sox9 and Snail, were also found to be repressed [76]. In another study, Wang et al. also detected significantly reduced expression levels of Pax6 and Ngn2 in Tet3 -/- mESCs generated by clustered regularly-interspaced short palindromic repeats (CRISPR) technology [68]. In a recent study, although NPCs could be induced efficiently from Tet3 knockout mESCs, they undergo apoptosis rapidly, and the terminal differentiation of neurons was greatly reduced [69]. These results together indicate the important roles of TET proteins in neurodevelopment. In addition, Gadd45b was also found to be involved in active demethylation, which was required for electroconvulsive treatments (ECTs)-induced adult neurogenesis [77,78]. We will discuss the role of Gadd45b in the section ‘DNA methylation dynamics in adult neurogenesis’.
DNA methylation dynamics in neurogenesis
DNA methylation dynamics during the differentiation of ESCs into NSCs/NPCs
Analyses of DNA modifications have revealed genome-wide 5mC differences between ESCs and NSCs. Meissner and colleagues validated high-throughput reduced representation bisulfite sequencing by sequencing MspI fragments from mouse ESCs and embryonic stem-derived NPCs. They found that approximately 8% of CpGs unmethylated in ESCs became largely methylated in NPCs, whereas approximately 2% of CpGs methylated in ESCs became unmethylated; these changes were strongly correlated with changes in histone methylation patterns, particularly in H3K4 [12]. Distal regulatory regions, including enhancers, silencers and boundary elements, account for most of these changes. In distal regulatory regions of the ESCs genome, H3K4me2 enrichment is related to low CpG methylation. This relationship was particularly strong for CpGs located in highly conserved noncoding elements. In NPCs, 75% of those H3K4me2-enriched sites in ESCs lost this mark, whereas numerous new H3K4me2 sites appeared, often in highly conserved noncoding element-rich regions surrounding activated developmental genes, such as Olig1 and Olig2. Loss or gain of H3K4 methylation was also inversely correlated with CpG methylation levels. These data suggest that 5mC undergoes substantial dynamic changes during ESC differentiation into NSCs, which is closely related to histone modification changes.
In a separate study, Stadler et al. revealed the orchestration between transcription factors and epigenetic changes during ESCs differentiation into NPCs [79]. They generated basepair-resolution mouse methylomes in stem cells and neuronal progenitors, and identified low-methylated regions (LMRs) with an average methylation of 30%, which were CpG-poor distal regulatory regions. LMRs were occupied by DNA-binding factors and their binding was necessary and sufficient to create LMRs. More importantly, they identified dramatic local changes in LMRs between ESCs and neuronal progenitors (NP). Cell-type-specific LMRs were occupied by cell-type-specific transcription factors, and LMR formation significantly correlated with increased expression of the nearest gene. In the case of NPCs, NP-specific LMRs were enriched for motifs of several neuronal transcription factors, such as Sox17 and Pax6, which could activate the expression of adjacent genes important for neuronal development. Interestingly, LMRs showed enrichment of 5hmC and TET1, raising the possibility that the different forms of cytosine oxidation products might be involved in this regulatory mechanism [79].
Using the newly developed comparative hydroxymethylated DNA immunoprecipitation (hMeDIP-seq) method, Tan et al. profiled and compared the DNA hydroxymethylomes of in vitro cultured mouse ESCs and mouse ESC-derived NPCs [67]. They revealed a dramatic loss of 5hmC (˜80% loss) during neural differentiation of mouse ESCs (Figure 3), whereas a small fraction of gene loci undergo de novo DNA hydroxymethylation. Many regions that undergone de novo DNA hydroxymethylation are located at genes associated with mature neuronal functions. In both ESCs and NPCs, there was a similar negative correlation between 5hmC density around translation start site regions and gene expression. On the other hand, in ESCs, genes with high expression levels displayed higher 5hmC enrichment in gene bodies than those with low expression levels. However, in NPCs, such a correlation was not obvious. Previous reports suggested significant 5hmC enrichment at enhancers in ESCs [80]. In NPCs, this 5hmC enrichment is lost in enhancers [67]. The functional potential of the DNA hydroxymethylation changes during neural differentiation merits future investigation.
During ESC differentiation, the regulation of Tet proteins is also important. The histone deacetylase, sirtuin 6 (SIRT6), which targets acetylated histone H3 at Lys 9 and 56 (H3K9ac and H3K56ac), represses the expression of pluripotent genes, Oct4 and Sox2, which in turn limits the expression of Tet1 and Tet2. The depletion of SIRT6 caused a derepression of Oct4 and Sox2, which triggers upregulation of Tet1 and Tet2 and a gain of 5hmC in neuroectoderm genes, including the Hoxa gene cluster, which is implicated in neural crest development, and Gata2 and Pax6, which are implicated in neurogenesis. Hyperhydromethylation leads to overexpression of neuroectoderm proteins, which results in skewed development toward neuroectoderm [81].
Methylation of DNA may result in the recruitment of methyl-CpG-binding proteins, such as MBD1 and MeCP2, which possess transcriptionally repressive functions [51]. It is intriguing to search and identify whether or not 5hmC can be recognized by such ‘readers’. Spruijt et al. applied large-scale quantitative mass spectrometry-based proteomics to identify 5mC- and 5hmC-binding proteins in ESCs, NPCs and the adult mouse brain. Uhrf2, an E3 ubiquitin protein ligase, which was not expressed in ESCs, was upregulated and highly expressed upon differentiation, and was identified as a specific 5hmC-binding protein in NPCs [82]. It would be interesting to study the role of specific Uhrf2–5hmC binding during ESC differentiation into NPCs.
DNA methylation dynamics in NPC differentiation into neurons
NPCs have the ability to generate all the cell types in the CNS, including neurons, astrocytes and oligodendrocytes. Hahn et al. investigated the in vivo global changes of 5mC and 5hmC between mouse neuronal progenitor cells (mNPCs) and daughter neurons [70]. While 5mC global levels remained unchanged, 5hmC levels increased during NPC differentiation into neurons, which is very interesting, as Tan et al. revealed a dramatic loss of 5hmC during mESC differentiation into NPCs (Figure 3) [67]. 5hmC was not enriched at enhancers in cortical NPCs and neurons [70], consistent with a previous report [67]. 5hmC associates preferentially with gene bodies of activated neuronal function-related genes, which are also characterized by loss of H3K27me3. Overexpression of Ezh2, a subunit of the Polycomb complex, which is responsible for the trimethylation of H3K27, or knockdown of Tet2 and Tet3, leads to defects in neural differentiation, suggesting that gain of 5hmC and loss of H3K27me3 cooperate to promote neurogenesis [70]. In another study, Colquitt et al. developed genomic maps of 5hmC along the developmental lineage of the main olfactory epithelium – from multipotent stem cells through neuronal progenitors to mature olfactory sensory neurons (mOSNs) [83]. During mOSN development, 5hmC increases over gene bodies, particularly between the progenitor and mOSN stages. Consistent with other studies [67,70], gene-body 5hmC levels positively correlate with gene expression. Overexpression of Tet3 led to a complex 5hmC change model, depending on control levels of 5hmC. Genes with high-level intragenic 5hmC showed a reduction of both 5hmC and expression level (such as Plxna1), and genes with moderate intragenic 5hmC showed an increase of 5hmC and expression level (such as Stxbp2). This change disrupted the normal molecular and anatomical features of the olfactory system [83]. Therefore, TET3 and its catalytic activity is crucial for the functional generation of mOSNs.
Due to the extremely low abundance of 5fC and 5caC in the genome, their functional roles in neurogenesis have not been systematically explored. Cortarzar et al. found that TDG KO neuronal progenitor cells failed to differentiate into neurons due to rapid loss of cell viability [84]. In another study, Wheldon et al. found 5fC and 5caC were transiently accumulated during both neuronal and glial differentiation of neural stem cells [85]. 5fC/5caC staining became weak and absent in three days after the induction of glial differentiation. After knockdown of TDG in NSCs, there were massive cell deaths during the initial two days of neuronal differentiation, consistent with Cortarzar’ work [84]. Surviving cells showed a high portion of 5fc/5caC staining [85]. The function of transient accumulation of 5fC/5caC in the initiation of NSC differentiation warrants further study.
DNA methylation dynamics in the neurogenic to astrogenic switch
As described above, NSCs at early gestation can only self-renew and then differentiate exclusively into neurons during mid-gestation. Gradually, NSCs begin to differentiate into glia (astrocyte and oligodendrocyte) at late-gestation [17]. Upon astrocytogenesis, astrocytic genes are activated by two signal pathways that act synergistically. The IL-6 family of cytokines (such as leukaemia inhibitory factor [LIF]) activates the JAK–STAT3 pathway, generating activated STAT3. BMPs activate downstream SMADs. Activated STAT3 and SMADs translocate to nucleus and form a complex bridged by the transcriptional coactivator p300/CBP. This complex binds to unmethylated promoters of astrogenic genes, such as Gfap (glial fibrillary acidic protein), and activate corresponding genes, thus switching neurogenesis to astrocytogenesis. In early- and mid-gestational NSCs, astrocytic gene promoters are hypermethylated, a status that impedes binding of the STAT3–p300/CBP–SMADs complex to their target sequence [86]. However, the mechanisms underlying the demethylation process of astrogenic genes are not yet fully elucidated. One of the potential mechanisms is passive demethylation due to the loss of methyltransferase activity of DNMT1. With conditional deletion of Dnmt1 from the neural lineage, mouse neural development was shown to be precociously shifted toward astroglial differentiation [87]. Further study showed Dnmt1 deletion caused demethylation not only in the STAT3-binding site in the Gfap promoter, but also gene promoters that are involved in the JAK–STAT pathway. The elevation of overall JAK–STAT signaling activity also contributed to precocious astroglial differentiation [87]. Another study showed that neuronal differentiation is involved in the demethylation of the Gfap promoter regions [88]. It was shown that Notch ligands could activate Notch signaling in the residual NSCs. Furthermore, forced expression of Notch intracellular domain prevents DNMT1 from binding to astrogenic gene promoters, thus accelerates demethylation [88]. Whether TET-mediated active demethylation is involved in this process remains to be determined.
DNA methylation dynamics in adult neurogenesis
The expression of DNMT3A is required for adult neurogenesis [89]. In _Dnmt3a_-null mouse, postnatal neurogenesis in both the V-SVZ and SGZ was impaired. Genome-wide analysis of postnatal NSCs indicates that DNMT3A occupies and methylates intergenic regions and gene bodies flanking proximal promoters of many neurogenesis-related genes, such as Dlx2, Sp8 and Neurog2. DNMT3A deletion led to downregulation of those genes. Surprisingly, DNMT3A-mediated nonproximal promoter methylation promoted neurogenic gene expression by inhibiting the binding of PRC2, which is responsible for the formation of repressive histone modification H3K27me3 [89]. Intriguingly, in a study discussed above [70], Hahn et al. found that, during the process of NPC differentiation into neurons, the 5mC undergoes little change, while H3K27me3 is lost or gained in many regions. Further, they found changes of 5hmC were negatively correlated with changes of H3K27me3 [70]. As Wu et al. [89] did not map the distribution change of 5hmC in their study, and _Dnmt3a_-null mouse may harbor less 5hmC, due to the loss of 5mC as a substrate, it remains possible that 5hmC, rather than 5mC, acts as the main DNA modification to antagonize Polycomb repression.
5mC ‘readers’, such as MBD1, act as important partners in adult neurogenesis. In vitro cultured MBD1 -/- adult neural stem cells (aNSCs) exhibited reduced neuronal differentiation and increased genomic instability. Furthermore, adult MBD1-/- mice showed consistent impaired neurogenesis and spatial learning [90]. Further study showed that the misregulation of miR184 may be involved in the underlying mechanism [91]. MBD1 can directly repress the generation of miR-184. MBD1 deficiency led to the derepression of miR-184, which binds to the 3’-UTR of Numblike (Numbl) mRNA, a known regulator of brain development, resulting in the low translation of Numbl. Low levels of Numbl promoted aNSC proliferation, but inhibited differentiation; overexpression of Numb1 can rescue the aNSC defects [91].
Adult neurogenesis can be activated by external stimuli. For example, synchronized activation of mature dentate neurons by ECT in adult mice caused sustained upregulation of hippocampal neurogenesis, without any detectable cell damage [77,92]. Gadd45b (growth arrest and DNA-damage-inducible protein 45) was strongly induced by ECT in mouse brain. Further, Gadd45b was indispensable for the DNA demethylation of specific promoters and expression of corresponding genes critical for adult neurogenesis, including brain-derived neurotrophic factor and FGF1. The Gadd45b knockout mouse exhibited specific deficits in ECT-induced proliferation of neural progenitors and dendritic growth of newborn neurons in the adult hippocampus. Further study revealed that TET1 and the AID/APOBEC family of cytidine deaminases were involved in ECT-induced DNA demethylation in the adult mouse dentate gyrus of the hippocampus [78]. TET1 mediates the generation of 5hmC, which undergoes deamination by AID/APOBEC to form 5hmU. 5hmU can be excised by 5hmU glycosylases (SMUG1, TDG, etc.) to generate an abasic site, which can undergo BER to be converted back to cytosine (C) [78].
TET protein is also involved in adult neurogenesis [73]. Mice lacking Tet1 showed impaired hippocampal neurogenesis and poor learning and memory. In adult neural progenitor cells derived from Tet1 KO mouse, a cohort of genes involved in progenitor proliferation were hypermethylated and downregulated, including Galanin, Ng2 and Ngb [73]. Additional studies on the other two Tet proteins are needed to fully understand the role of TET proteins in adult neurogenesis.
Conclusion
Unlike extracellular signals and transcription factors, which have been well-studied for many years, epigenetic regulations, especially DNA methylation, in mammalian neurogenesis have only attracted attentions in recent years. Various types of cells involved in neurogenesis process in an organism share the same genome, being derived from the same zygote, emphasizing the key roles of epigenetic regulations. As mentioned above, dysregulation of DNA methylation ‘writers’, ‘erasers’ and ‘readers’ could result in neurogenesis and neurodevelopment deficit or even lethality, suggesting the important role of DNA methylation in nervous system. Genome-wide profiling revealed DNA methylation dynamics during neural lineage commitment, within which the change of 5hmC level was particularly significant [67,70]. DNA methylation dynamics correlates with the change of histone methylation, as well as the expression of related genes [12,70,83]. It is possible that different DNA methylation states are important to define cell identity. In fact, a recent study has shown that three subtypes of neocortical neurons had highly distinctive epigenomic landscapes [93]. Due to the relatively small amount of studies, it is still early to conclude that DNA methylation dynamics plays a central role in neurogenesis. Nevertheless, it is undoubted that epigenetic regulations (including DNA methylation), extracellular signals and transcription factors work together to assure the proper neurogenesis process.
Future perspective
Understanding the role of dynamic DNA methylation in neurogenesis is still in its infancy. Genome-wide maps of different cytosine modifications at single-base solution during the neural lineage commitment, from ESCs to NSCs to neurons, are clearly needed for future studies. In addition, given the complexity and heterogeneity of neurogenesis, the approaches of epigenomic and gene expression analyses at the single-cell level must be developed, as well.
Besides TDG and the BER pathway, which are validated biochemically by multiple studies [9,10], there are many putative mechanisms of active erasure of 5-mC (for review, see [54]), as TDG and the BER pathway cannot explain all active demethylation processes; one example is the pronuclei demethylation in the zygote. On the other hand, although some studies have identified some 5hmC ‘readers’ [82], it remains to be determined whether these ‘readers’ can specially recognize modified cytosine or simply bind to hydroxyl, formyl or carboxyl group. It would be intriguing to study the ‘eraser’ and ‘reader’ in methylation dynamics during neurogenesis.
In addition, adenine N6-methylation (6mA) was previously thought to exist only in bacteria, protists and other lower eukaryotes [94]. Recently, however, several papers revealed that 6mA exists at considerable levels in Chlamydomonas [95], Caenorhabditis elegans [96] and Drosophila [97]. 6mA is related to gene activation [95], differentiation of early germ cells and transposon expression [97]. Intriguingly, DMAD, a Tet analog in fly, was identified as 6mA demethylase [97]. It would be interesting to explore whether 6mA is present in mammalian cells, as well as the role of 6mA in gene regulation, particularly, in the context of neurogenesis.
Executive summary.
Fundamentals of neurogenesis
- Apical radial glials function as neural stem cells and generate almost all neurons, astrocytes and oligodendrocytes sequentially during embryonic neurogenesis.
- Neural progenitor cells in the adult ventricular-subventricular zone and subgranular zone continually generate neurons throughout the life.
- Extracellular signals, intrinsic regulators and epigenetic modifiers synergistically orchestrate the complex neurogenesis process in both the embryo and adult stage.
DNA methylation & demethylation
- The DNA methyltransferases (DNMT) family methylates cytosine (C) to 5mC. TET family proteins catalyze the oxidation of mC along the mC-5hmC-5fC-5caC pathway. 5caC can be excised by thymine DNA glycosylase, and then be repaired to a cytosine via the base excision repair pathway.
DNA methylation writers in neurogenesis
- DNMTs and ten-eleven translocations are expressed differently in various stages and locations related to neurogenesis.
- Perturbation of DNMTs and ten-eleven translocations leads to impaired neurogenesis.
DNA methylation dynamics during neurogenesis
- Global DNA methylation state changes in the neural lineage commitment.
- Changes of DNA methylation are related to other epigenetic marks, including histone modifications.
- Epigenetic dynamics cause the expression changes of corresponding genes.
Future perspective
- Fully understanding DNA methylation dynamics in neurogenesis requires comprehensive analysis of different forms of cytosine modification along with neural lineage.
- Identifying the ‘erasers’ and ‘readers’ will facilitate methylation dynamics research.
- Whether the newly identified 6mA plays a role in neurogenesis warrants further study.
Acknowledgements
The authors would like to thank C Strauss for critical reading of the manuscript.
Footnotes
Financial & competing interests disclosure
The authors were supported in part by grants from the NIH (NS079625, NS05160 and MH102690 to P Jin). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Taverna E, Gotz M, Huttner WB. The cell biology of neurogenesis: toward an understanding of the development and evolution of the neocortex. Annu. Rev. Cell Dev. Biol. 2014;30:465–502. doi: 10.1146/annurev-cellbio-101011-155801. [DOI] [PubMed] [Google Scholar]
- 2.Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russo VEA, Martienssen RA, Riggs AD, et al. In: Epigenetic Mechanisms of Gene Regulation. Russo VEA, Martienssen RA, Riggs AD, editors. Cold Spring Harbor Laboratory Press; Woodbury, NY, USA: 1996. [Google Scholar]
- 4.Bird A. Perceptions of epigenetics. Nature. 2007;447(7143):396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- 5.Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324(5929):929–930. doi: 10.1126/science.1169786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tahiliani M, Koh KP, Shen Y, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324(5929):930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito S, D'Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466(7310):1129–1133. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ficz G, Branco MR, Seisenberger S, et al. Dynamic regulation of 5-hydroxymethylcytosine in mouse ES cells and during differentiation. Nature. 2011;473(7347):398–402. doi: 10.1038/nature10008. [DOI] [PubMed] [Google Scholar]
- 9.He YF, Li BZ, Li Z, et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science. 2011;333(6047):1303–1307. doi: 10.1126/science.1210944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito S, Shen L, Dai Q, et al. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333(6047):1300–1303. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pastor WA, Pape UJ, Huang Y, et al. Genome-wide mapping of 5-hydroxymethylcytosine in embryonic stem cells. Nature. 2011;473(7347):394–397. doi: 10.1038/nature10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meissner A, Mikkelsen TS, Gu H, et al. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature. 2008;454(7205):766–770. doi: 10.1038/nature07107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegel A, Sapru HN. Essential Neuroscience. Wolters Kluwer; MD, PA, USA: 2015. pp. 19–32. [Google Scholar]
- 14.Gotz M, Huttner WB. The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 2005;6(10):777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- 15.Gotz M, Barde YA. Radial glial cells defined and major intermediates between embryonic stem cells and CNS neurons. Neuron. 2005;46(3):369–372. doi: 10.1016/j.neuron.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Mori T, Buffo A, Gotz M. The novel roles of glial cells revisited: the contribution of radial glia and astrocytes to neurogenesis. Curr. Top. Dev. Biol. 2005;69:67–99. doi: 10.1016/S0070-2153(05)69004-7. [DOI] [PubMed] [Google Scholar]
- 17.Qian X, Shen Q, Goderie SK, et al. Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron. 2000;28(1):69–80. doi: 10.1016/s0896-6273(00)00086-6. [DOI] [PubMed] [Google Scholar]
- 18.Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat. Rev. Neurosci. 2007;8(6):427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- 19.Sauvageot CM, Stiles CD. Molecular mechanisms controlling cortical gliogenesis. Curr. Opin. Neurobiol. 2002;12(3):244–249. doi: 10.1016/s0959-4388(02)00322-7. [DOI] [PubMed] [Google Scholar]
- 20.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge WP, Miyawaki A, Gage FH, Jan YN, Jan LY. Local generation of glia is a major astrocyte source in postnatal cortex. Nature. 2012;484(7394):U376–U381. doi: 10.1038/nature10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki IK, Vanderhaeghen P. Is this a brain which I see before me? Modeling human neural development with pluripotent stem cells. Development. 2015;142(18):3138–3150. doi: 10.1242/dev.120568. [DOI] [PubMed] [Google Scholar]
- 23.Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18(9):383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- 24.Zappaterra MW, Lehtinen MK. The cerebrospinal fluid: regulator of neurogenesis, behavior, and beyond. Cell. Mol. Life Sci. 2012;69(17):2863–2878. doi: 10.1007/s00018-012-0957-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Huang CT, Chen J, et al. Pax6 is a human neuroectoderm cell fate determinant. Cell Stem Cell. 2010;7(1):90–100. doi: 10.1016/j.stem.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamby ME, Coskun V, Sun YE. Transcriptional regulation of neuronal differentiation: the epigenetic layer of complexity. Biochim. Biophys. Acta. 2008;1779(8):432–437. doi: 10.1016/j.bbagrm.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ballas N, Grunseich C, Lu DD, Speh JC, Mandel G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell. 2005;121(4):645–657. doi: 10.1016/j.cell.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Codega P, Silva-Vargas V, Paul A, et al. Prospective identification and purification of quiescent adult neural stem cells from their in vivo niche. Neuron. 2014;82(3):545–559. doi: 10.1016/j.neuron.2014.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lazarini F, Lledo PM. Is adult neurogenesis essential for olfaction? Trends Neurosci. 2011;34(1):20–30. doi: 10.1016/j.tins.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70(4):589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergmann O, Spalding KL, Frisen J. Adult neurogenesis in humans. Cold Spring Harb. Perspect. Biol. 2015;7(7):a018994. doi: 10.1101/cshperspect.a018994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spalding KL, Bergmann O, Alkass K, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153(6):1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imayoshi I, Sakamoto M, Yamaguchi M, Mori K, Kageyama R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J. Neurosci. 2010;30(9):3489–3498. doi: 10.1523/JNEUROSCI.4987-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pierfelice T, Alberi L, Gaiano N. Notch in the vertebrate nervous system: an old dog with new tricks. Neuron. 2011;69(5):840–855. doi: 10.1016/j.neuron.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 35.Nam HS, Benezra R. High levels of Id1 expression define B1 type adult neural stem cells. Cell Stem Cell. 2009;5(5):515–526. doi: 10.1016/j.stem.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim DA, Huang YC, Swigut T, et al. Chromatin remodelling factor Mll1 is essential for neurogenesis from postnatal neural stem cells. Nature. 2009;458(7237):529–533. doi: 10.1038/nature07726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386(6624):493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 38.Van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma DK, Marchetto MC, Guo JU, Ming GL, Gage FH, Song H. Epigenetic choreographers of neurogenesis in the adult mammalian brain. Nat. Neurosci. 2010;13(11):1338–1344. doi: 10.1038/nn.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 2010;11(3):204–220. doi: 10.1038/nrg2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee HJ, Hore TA, Reik W. Reprogramming the methylome: erasing memory and creating diversity. Cell Stem Cell. 2014;14(6):710–719. doi: 10.1016/j.stem.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lister R, Mukamel EA, Nery JR, et al. Global epigenomic reconfiguration during mammalian brain development. Science. 2013;341(6146):1237905. doi: 10.1126/science.1237905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shirane K, Toh H, Kobayashi H, et al. Mouse oocyte methylomes at base resolution reveal genome-wide accumulation of non-CpG methylation and role of DNA methyltransferases. PLoS Genet. 2013;9(4):e1003439. doi: 10.1371/journal.pgen.1003439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okano M, Xie S, Li E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat. Genet. 1998;19(3):219–220. doi: 10.1038/890. [DOI] [PubMed] [Google Scholar]
- 45.Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99(3):247–257. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 46.Goll MG, Bestor TH. Eukaryotic cytosine methyltransferases. Annu. Rev. Biochem. 2005;74:481–514. doi: 10.1146/annurev.biochem.74.010904.153721. [DOI] [PubMed] [Google Scholar]
- 47.Bestor TH. The DNA methyltransferases of mammals. Hum. Mol. Genet. 2000;9(16):2395–2402. doi: 10.1093/hmg/9.16.2395. [DOI] [PubMed] [Google Scholar]
- 48.Bostick M, Kim JK, Esteve PO, Clark A, Pradhan S, Jacobsen SE. UHRF1 plays a role in maintaining DNA methylation in mammalian cells. Science. 2007;317(5845):1760–1764. doi: 10.1126/science.1147939. [DOI] [PubMed] [Google Scholar]
- 49.Smith ZD, Meissner A. DNA methylation: roles in mammalian development. Nat. Rev. Genet. 2013;14(3):204–220. doi: 10.1038/nrg3354. [DOI] [PubMed] [Google Scholar]
- 50.Tate PH, Bird AP. Effects of DNA methylation on DNA-binding proteins and gene expression. Curr. Opin. Genet. Dev. 1993;3(2):226–231. doi: 10.1016/0959-437x(93)90027-m. [DOI] [PubMed] [Google Scholar]
- 51.Defossez PA, Stancheva I. Biological functions of methyl-CpG-binding proteins. Prog. Mol. Biol. Transl. Sci. 2011;101:377–398. doi: 10.1016/B978-0-12-387685-0.00012-3. [DOI] [PubMed] [Google Scholar]
- 52.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16(1):6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 53.Mayer W, Niveleau A, Walter J, Fundele R, Haaf T. Demethylation of the zygotic paternal genome. Nature. 2000;403(6769):501–502. doi: 10.1038/35000656. [DOI] [PubMed] [Google Scholar]
- 54.Wu H, Zhang Y. Reversing DNA methylation: mechanisms, genomics, and biological functions. Cell. 2014;156(1–2):45–68. doi: 10.1016/j.cell.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maiti A, Drohat AC. Thymine DNA glycosylase can rapidly excise 5-formylcytosine and 5-carboxylcytosine potential implications for active demethylation of CpG sites. J. Biol. Chem. 2011;286(41):35334–35338. doi: 10.1074/jbc.C111.284620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gu TP, Guo F, Yang H, et al. The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. Nature. 2011;477(7366):606–610. doi: 10.1038/nature10443. [DOI] [PubMed] [Google Scholar]
- 57.Inoue A, Shen L, Dai Q, He C, Zhang Y. Generation and replication-dependent dilution of 5fC and 5caC during mouse preimplantation development. Cell Res. 2011;21(12):1670–1676. doi: 10.1038/cr.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iqbal K, Jin SG, Pfeifer GP, Szabo PE. Reprogramming of the paternal genome upon fertilization involves genome-wide oxidation of 5-methylcytosine. Proc. Natl Acad. Sci. USA. 2011;108(9):3642–3647. doi: 10.1073/pnas.1014033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Santos F, Peat J, Burgess H, Rada C, Reik W, Dean W. Active demethylation in mouse zygotes involves cytosine deamination and base excision repair. Epigenet. Chromatin. 2013;6(1):39. doi: 10.1186/1756-8935-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pastor WA, Aravind L, Rao A. TETonic shift: biological roles of TET proteins in DNA demethylation and transcription. Nat. Rev. Mol. Cell Biol. 2013;14(6):341–356. doi: 10.1038/nrm3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goto K, Numata M, Komura JI, Ono T, Bestor TH, Kondo H. Expression of DNA methyltransferase gene in mature and immature neurons as well as proliferating cells in mice. Differentiation. 1994;56(1–2):39–44. doi: 10.1046/j.1432-0436.1994.56120039.x. [DOI] [PubMed] [Google Scholar]
- 62.Feng J, Chang H, Li E, Fan G. Dynamic expression of de novo DNA methyltransferases Dnmt3a and Dnmt3b in the central nervous system. J. Neurosci. Res. 2005;79(6):734–746. doi: 10.1002/jnr.20404. [DOI] [PubMed] [Google Scholar]
- 63.Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69(6):915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 64.Fan G, Beard C, Chen RZ, et al. DNA hypomethylation perturbs the function and survival of CNS neurons in postnatal animals. J. Neurosci. 2001;21(3):788–797. doi: 10.1523/JNEUROSCI.21-03-00788.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rhee KD, Yu J, Zhao CY, Fan G, Yang XJ. Dnmt1-dependent DNA methylation is essential for photoreceptor terminal differentiation and retinal neuron survival. Cell Death Dis. 2012;3:e427. doi: 10.1038/cddis.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nguyen S, Meletis K, Fu D, Jhaveri S, Jaenisch R. Ablation of de novo DNA methyltransferase Dnmt3a in the nervous system leads to neuromuscular defects and shortened lifespan. Dev. Dyn. 2007;236(6):1663–1676. doi: 10.1002/dvdy.21176. [DOI] [PubMed] [Google Scholar]
- 67.Tan L, Xiong L, Xu W, et al. Genome-wide comparison of DNA hydroxymethylation in mouse embryonic stem cells and neural progenitor cells by a new comparative hMeDIP-seq method. Nucleic Acids Res. 2013;41(7):e84. doi: 10.1093/nar/gkt091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y, Zhang Y. Regulation of TET protein stability by calpains. Cell Rep. 2014;6(2):278–284. doi: 10.1016/j.celrep.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li T, Yang D, Li J, Tang Y, Yang J, Le W. Critical role of Tet3 in neural progenitor cell maintenance and terminal differentiation. Mol. Neurobiol. 2015;51(1):142–154. doi: 10.1007/s12035-014-8734-5. [DOI] [PubMed] [Google Scholar]
- 70.Hahn MA, Qiu R, Wu X, et al. Dynamics of 5-hydroxymethylcytosine and chromatin marks in Mammalian neurogenesis. Cell Rep. 2013;3(2):291–300. doi: 10.1016/j.celrep.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dawlaty MM, Ganz K, Powell BE, et al. Tet1 is dispensable for maintaining pluripotency and its loss is compatible with embryonic and postnatal development. Cell Stem Cell. 2011;9(2):166–175. doi: 10.1016/j.stem.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dawlaty MM, Breiling A, Le T, et al. Combined deficiency of Tet1 and Tet2 causes epigenetic abnormalities but is compatible with postnatal development. Dev. Cell. 2013;24(3):310–323. doi: 10.1016/j.devcel.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang RR, Cui QY, Murai K, et al. Tet1 regulates adult hippocampal neurogenesis and cognition. Cell Stem Cell. 2013;13(2):237–245. doi: 10.1016/j.stem.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rudenko A, Dawlaty MM, Seo J, et al. Tet1 is critical for neuronal activity-regulated gene expression and memory extinction. Neuron. 2013;79(6):1109–1122. doi: 10.1016/j.neuron.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaas GA, Zhong C, Eason DE, et al. TET1 controls CNS 5-methylcytosine hydroxylation, active DNA demethylation, gene transcription, and memory formation. Neuron. 2013;79(6):1086–1093. doi: 10.1016/j.neuron.2013.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu Y, Xu C, Kato A, et al. Tet3 CXXC domain and dioxygenase activity cooperatively regulate key genes for Xenopus eye and neural development. Cell. 2012;151(6):1200–1213. doi: 10.1016/j.cell.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ma DK, Jang MH, Guo JU, et al. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323(5917):1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guo JU, Su YJ, Zhong C, Ming GL, Song HJ. Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell. 2011;145(3):423–434. doi: 10.1016/j.cell.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stadler MB, Murr R, Burger L, et al. DNA-binding factors shape the mouse methylome at distal regulatory regions. Nature. 2011;480(7378):490–495. doi: 10.1038/nature10716. [DOI] [PubMed] [Google Scholar]
- 80.Yu M, Hon GC, Szulwach KE, et al. Base-resolution analysis of 5-hydroxymethylcytosine in the mammalian genome. Cell. 2012;149(6):1368–1380. doi: 10.1016/j.cell.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Etchegaray JP, Chavez L, Huang Y, et al. The histone deacetylase SIRT6 controls embryonic stem cell fate via TET-mediated production of 5-hydroxymethylcytosine. Nat. Cell Biol. 2015;17(5):545–557. doi: 10.1038/ncb3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spruijt CG, Gnerlich F, Smits AH, et al. Dynamic readers for 5-(hydroxy)methylcytosine and its oxidized derivatives. Cell. 2013;152(5):1146–1159. doi: 10.1016/j.cell.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 83.Colquitt BM, Allen WE, Barnea G, Lomvardas S. Alteration of genic 5-hydroxymethylcytosine patterning in olfactory neurons correlates with changes in gene expression and cell identity. Proc. Natl Acad. Sci. USA. 2013;110(36):14682–14687. doi: 10.1073/pnas.1302759110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cortazar D, Kunz C, Selfridge J, et al. Embryonic lethal phenotype reveals a function of TDG in maintaining epigenetic stability. Nature. 2011;470(7334):419–423. doi: 10.1038/nature09672. [DOI] [PubMed] [Google Scholar]
- 85.Wheldon LM, Abakir A, Ferjentsik Z, et al. Transient accumulation of 5-carboxylcytosine indicates involvement of active demethylation in lineage specification of neural stem cells. Cell. Rep. 2014;7(5):1353–1361. doi: 10.1016/j.celrep.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 86.Takizawa T, Nakashima K, Namihira M, et al. DNA methylation is a critical cell-intrinsic determinant of astrocyte differentiation in the fetal brain. Dev. Cell. 2001;1(6):749–758. doi: 10.1016/s1534-5807(01)00101-0. [DOI] [PubMed] [Google Scholar]
- 87.Fan G, Martinowich K, Chin MH, et al. DNA methylation controls the timing of astrogliogenesis through regulation of JAK–STAT signaling. Development. 2005;132(15):3345–3356. doi: 10.1242/dev.01912. [DOI] [PubMed] [Google Scholar]
- 88.Namihira M, Kohyama J, Semi K, et al. Committed neuronal precursors confer astrocytic potential on residual neural precursor cells. Dev. Cell. 2009;16(2):245–255. doi: 10.1016/j.devcel.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 89.Wu H, Coskun V, Tao J, et al. Dnmt3a-dependent nonpromoter DNA methylation facilitates transcription of neurogenic genes. Science. 2010;329(5990):444–448. doi: 10.1126/science.1190485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao XY, Ueba T, Christie BR, et al. Mice lacking methyl-CpG binding protein 1 have deficits in adult neurogenesis and hippocampal function. Proc. Natl Acad. Sci. USA. 2003;100(11):6777–6782. doi: 10.1073/pnas.1131928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu C, Teng ZQ, Santistevan NJ, et al. Epigenetic regulation of miR-184 by MBD1 governs neural stem cell proliferation and differentiation. Cell Stem Cell. 2010;6(5):433–444. doi: 10.1016/j.stem.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingstrom A. Increased neurogenesis in a model of electroconvulsive therapy. Biol. Psychiatry. 2000;47(12):1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- 93.Mo A, Mukamel EA, Davis FP, et al. Epigenomic signatures of neuronal diversity in the mammalian brain. Neuron. 2015;86(6):1369–1384. doi: 10.1016/j.neuron.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wion D, Casadesus J. N6-methyl-adenine: an epigenetic signal for DNA–protein interactions. Nat. Rev. Microbiol. 2006;4(3):183–192. doi: 10.1038/nrmicro1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fu Y, Luo GZ, Chen K, et al. N6-methyldeoxyadenosine marks active transcription start sites in Chlamydomonas . Cell. 2015;161(4):879–892. doi: 10.1016/j.cell.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Greer EL, Blanco MA, Gu L, et al. DNA Methylation on N6-adenine in C. elegans . Cell. 2015;161(4):868–878. doi: 10.1016/j.cell.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang G, Huang H, Liu D, et al. N6-methyladenine DNA modification in Drosophila . Cell. 2015;161(4):893–906. doi: 10.1016/j.cell.2015.04.018. [DOI] [PubMed] [Google Scholar]