Lightning and charge processes in brown dwarf and exoplanet atmospheres (original) (raw)
Abstract
The study of the composition of brown dwarf atmospheres helped to understand their formation and evolution. Similarly, the study of exoplanet atmospheres is expected to constrain their formation and evolutionary states. We use results from three-dimensional simulations, kinetic cloud formation and kinetic ion-neutral chemistry to investigate ionization processes that will affect their atmosphere chemistry: the dayside of super-hot Jupiters is dominated by atomic hydrogen, and not H2O. Such planetary atmospheres exhibit a substantial degree of thermal ionization and clouds only form on the nightside where lightning leaves chemical tracers (e.g. HCN) for possibly long enough to be detectable. External radiation may cause exoplanets to be enshrouded in a shell of highly ionized, H3+-forming gas and a weather-driven aurora may emerge. Brown dwarfs enable us to study the role of electron beams for the emergence of an extrasolar, weather system-driven aurora-like chemistry, and the effect of strong magnetic fields on cold atmospheric gases. Electron beams trigger the formation of H3+ in the upper atmosphere of a brown dwarf (e.g. LSR-J1835), which may react with it to form hydronium, H3O+, as a longer lived chemical tracer. Brown dwarfs and super-hot gas giants may be excellent candidates to search for H3O+ as an H3+ product.
This article is part of a discussion meeting issue ‘Advances in hydrogen molecular ions: H3+, H5+ and beyond’.
Keywords: exoplanet, brown dwarf, lightning, charges, aurora
1. Introduction
Exoplanet science is moving from object discoveries (e.g. [1]) into characterization and analysis of the discovered objects (e.g. [2–6]). Transit spectroscopy has shown that exoplanet atmospheres form clouds (e.g. [7]), and that phase curves of exoplanets indicate the presence of winds driven by the external, host-star irradiation (e.g. [8,9]). The formation of extrasolar clouds and their time-variability has first been analysed in brown dwarf atmospheres [10–12], which are repeatedly studied as exoplanet analogues [13]. Complex models have been developed for brown dwarf and planetary atmospheres, their chemical composition and the clouds that form [14–18].
In fact, clouds have been found to be quite frustrating as they are blocking our view into the extrasolar and chemically very different atmospheres of exoplanets where one might hope to find the signature of biomolecules or the precursors thereof. Because the atmospheric chemistry of exoplanets (and brown dwarfs) is very different to Earth, the clouds that form are not made of water only, but are made of a mix of minerals and oxides in the hotter exoplanets in addition to water or methane clouds in cooler exoplanets and brown dwarfs [19]. Although the Solar System gas planet atmospheres appear less dynamic compared with some of the extrasolar gas giants, lightning events are detected directly in the cloudy atmospheres of Earth, Jupiter and Saturn, are debatable for Venus, and indirectly inferred for Neptune and Uranus. Sprites and high-energy particles are observed above thunderclouds on Earth [20,21], and are predicted for Jupiter, Saturn and Venus. Lightning observations can only be conducted in situ on Earth such that the lightning statistics for all other Solar System planets are rather incomplete [22]. Possible analogues for exoplanet lightning or lightning in brown dwarfs are terrestrial volcanoes that produce lightning during an eruption in their plumes. Studying lightning in other planets inside and outside our Solar System is of interest because it enables us to study the electrostatic character of such alien atmospheres, and it opens up new possibilities for tracking the dynamic behaviour and the associated chemical changes in such extraterrestrial environments. Other astronomical objects, brown dwarfs and planet-forming disks, are also expected to have lightning discharges [23,24] because the underlying physical regimes are similar [25].
Charge processes on exoplanets and brown dwarfs are furthermore driven by their environments. The global environment is very different for exoplanets and for brown dwarfs, reflecting their different formation mechanisms. The exoplanet's atmosphere is exposed to the radiation field of its host star and the effect of it will also depend on the exoplanet's size, mass, atmosphere thickness and its distance from the host star. A seemingly small difference in orbital distance and planetary mass leads to vastly different atmospheric conditions, deciding between habitable (Earth), poisoning (Venus) or too extreme (Mars) for life as we know it. A brown dwarf is exposed to the interstellar radiation field, or when being part of a binary system with a white dwarf, it may suffer the harsh radiation from the nearby white dwarf [26,27]. In contrast to planets, brown dwarfs are magnetically very active with B ≈ 103G [28], and a current system may cause electrons to collide with the atmospheric hydrogen. The highly polarized kHz and MHz radio emission has been interpreted as Auroral emission, comparable with Jupiter but 104 × stronger [29].
Predictions of significant H3+ concentrations in Jupiter's upper atmosphere [30,31] led to observational programmes that successfully detected H3+ in Jupiter in situ [32] and remotely [33]. Likewise, the ionization of exoplanet atmospheres is predicted to lead to the formation of upper atmospheric H3+ [34], and this prediction has led to several observational programmes to search for H3+ in Hot Jupiter atmospheres, thus far without success [35–37]. Although there are spectral hints of H3+ in brown dwarf atmospheres [38], no definitive detection of H3+ has been made in a brown dwarf atmosphere, and there have been as of yet no published results from a programme to search for H3+ in brown dwarf atmospheres. We will allude to why it may be difficult to detect H3+ on the targeted exoplanets and brown dwarfs in this paper.
In what follows, we discuss environmental processes that affect the ionization of exoplanet and brown dwarf atmospheres that cause the formation of a global or partial ionosphere (§2). The term ‘aurora’ is used for a range of processes involving accelerated electrons in the upper atmosphere of the Solar System planets. Here, an aurora is understood to be associated with a pool of free charges, a confining field and collisional partners for accelerated charges. An ionosphere, therefore, provides one of the necessary conditions for an aurora to emerge on brown dwarfs and exoplanets. The conditions for lightning that locally changes the thermodynamic conditions dramatically, and that may leave some tracer molecules, are laid out for exoplanets and brown dwarfs in §§2c. We proceed to introduce how extrasolar aurorae might be traced by chemical signatures beyond H3+, namely by hydronium, H3O+ in §3. We suggest brown dwarfs as optimal candidates to search for H3O+ as a product of H3+.
2. Ionization processes on exoplanets and brown dwarfs
The best-studied exoplanets to date are the short-period giant gas planets HD 189733b and HD 209458b, and super-hot gas giants like WASP-18b, WASP-121b and HAT-P-7b (see [18,39–42] and references therein). These exoplanets, despite being clearly uninhabitable, enable us to test our models and expand our ideas into the extreme regimes of exoplanetary atmospheres. For example, molecules as tracers for atmospheric processes have long been studied in cool stars (see [43] for a review), and the respective model atmosphere expertise is the backbone of exoplanet and brown dwarf atmosphere research [44,45]. Low-gravity brown dwarfs turn out to be rather suitable analogues for long-period extrasolar giant gas planets and almost overlap in the colour-magnitude diagram (see fig. 1 in [46]). White dwarf-brown dwarf binaries can be seen as analogues to short-period giant gas planets [27].
The ionization processes in brown dwarf and exoplanet atmospheres are determined by their individual environments and more locally by the objects' character (size, mass, age) itself. The dominating ionization processes in exoplanets and brown dwarfs are thermal ionisation (Brownian motion) of the gas, tribolelectric charging (turbulent motion) of cloud articles, and photoionization, which can affect both gas and cloud particles. Lightning and Alfvén ionization produce short-lived population of charges in the atmosphere.
(a). Thermal ionization and the day/night differences on ultra-hot Jupiters
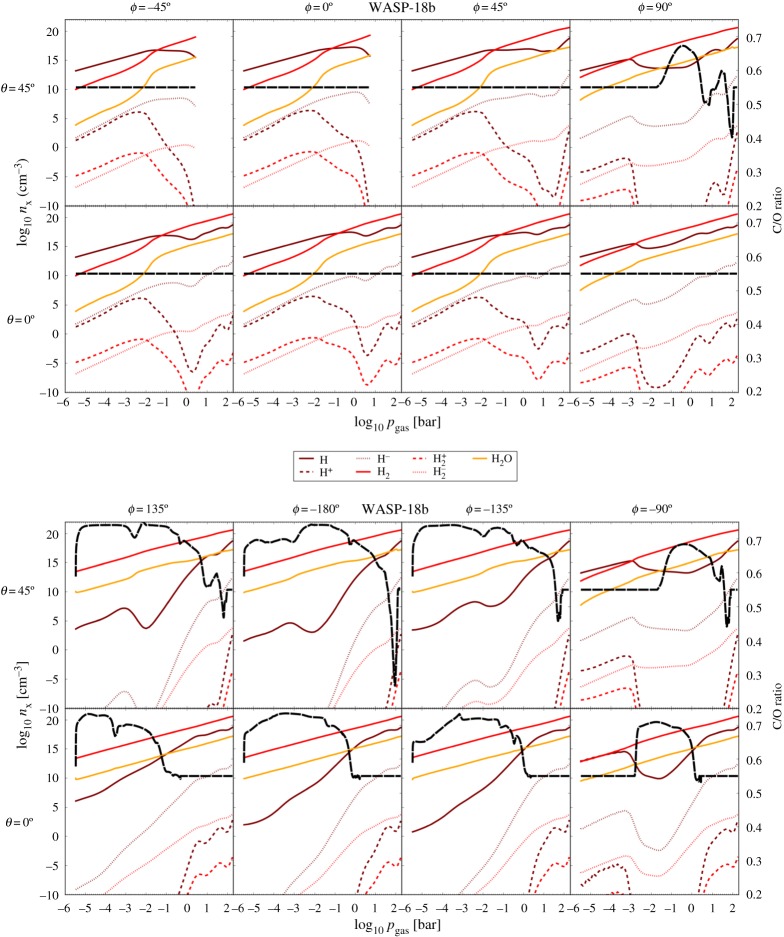
The high irradiation that close-in planets with a rather small orbital distance to their host star receive can lead to a drastic temperature difference between dayside and nightside. That results in large pressure gradients which drive a strong global circulation. For example, the three-dimensional global circulation model for WASP-18b from [40] suggests _T_day − _T_night ≈ 2500 K (figure 1), but global circulation can also be driven by more moderate day-night differences [47,48]. As shown in figure 1 for the super-hot Jupiter WASP-18b, the vertical dayside temperature profiles (ϕ = − 45°, 0°, 45°) have strong temperature inversions, i.e. outward increasing gas temperatures, which become more shallow in the terminator regions (ϕ = 90°, −90°) where they occur at lower pressures compared with the dayside. The nightside temperature profiles (ϕ = 135°, −180°, −135°) smoothly decrease outwards, and are very similar to those of non-irradiated brown dwarfs and planets that orbit their host star at a large distance (so-called directly imaged planets). The effect of the high irradiation appears smaller for higher latitudes (θ = 45°, dashed lines in figure 1). The depth of the temperature inversions is less at higher latitudes and the day-night temperature difference is smaller than in the equatorial regions.
Figure 1.
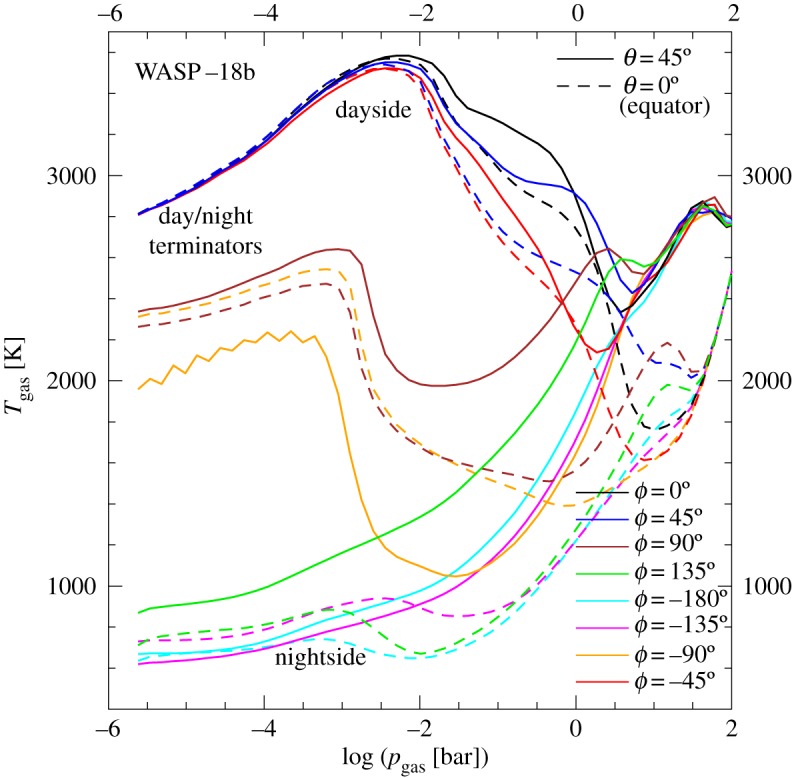
Highly irradiated, ultra-hot Jupiters develop extreme temperature differences of 2500 K between dayside (ϕ = − 45°, 0°, 45°) and nightside (ϕ = 135°, −180°, − 135°). An ionosphere may emerge on the dayside, and mineral clouds form on the nightside [39]. The terminator regions (ϕ = 90°, −90°) are transition regions between the two extreme atmosphere conditions. The one-dimensional profiles are from a cloud-free three-dimensional GCM simulation [40]. (Online version in colour.)
The extreme day-night temperature differences produce very different chemical structures of the dayside and the nightside, and transition regions at the terminators (figure 2). On the dayside (top), all H2 is thermally dissociated and the atmosphere is dominated by the atomic hydrogen (H). No H2O can form because of the high temperatures. Atoms like Na, K and Mg but also Al and Ti undergo thermal ionization causing the local degree of thermal ionization to change by an order of 12 magnitudes from the night- to a dayside value of 10−3.5. The plasma frequency, _ω_pe∼(_n_e/_m_e)1/2, is 104 × the electron-neutral collision frequency, _ν_ne∼_n_gas_v_them, e, hence, the electromagnetic interactions dominate over kinetic collisions between the electrons and neutrals. A current system similar to that in the Earth's atmosphere may be expected and a weather-driven aurora may emerge if the planet possesses a confining field. On the cold nightside (figure 2, bottom), H2 remains the dominating gas species with also no other species being thermally ionized in the upper, cool part of the atmosphere. The next most abundant H-binding molecule after H2 is H2O. Cloud formation causes a depletion of oxygen which causes the carbon-to-oxygen ratio, C/O, to increase to greater than 0.7 [39]. Figure 2 depicts C/O as a tracer for the cloud location in the atmosphere (black dashed line). Lightning activity can be expected on the cloud-forming nightside of super-hot gas giants and the photoionization of H2 may produce H3+ because of a very low degree of ionization and a slow dissociative recombination of H3+ (see §§3b).
Figure 2.
The changing number densities (_n_x [cm−3], left axis) of hydrogen-binding gas species from the dayside (top) to the nightside (bottom). C/O is shown as a black dashed line (right axis) to demonstrate where cloud formation takes place. The dayside is made of a cloud-free, H-dominated gas and the nightside is made of a H2-dominated gas with vivid cloud formation (C/O > C/Osolar). The extension of the cloud in pressure space (_x_-axis) changes between equator (θ = 0) and northern hemisphere (θ = 45°) [39,40]. (Online version in colour.)
(b). H3+ in an envelope of highly ionized gas on brown dwarfs?
The radiative environment of exoplanets and brown dwarfs affect the atmospheric chemistry due to photon-chemistry processes but also due to thermal processes. Exoplanets are always exposed to the radiation field of their host stars (the flux of which scales with r_−2, r being the star–planet distance), and it will be the harsh interstellar radiation field or even the radiation from a white dwarf that affects a brown dwarf's atmosphere [38]. The white dwarfs' radiation field dissociates H2 in the upper atmosphere of brown dwarfs with observed emission from H_α, He, Na, Mg, Si, K, Ca, Ti and Fe [26]. Photo-chemical processes triggered by Lyman continuum radiation further dissociates atomic hydrogen. A completely ionized outer atmosphere environment results for brown dwarfs as white dwarf companions, and to a lesser degree for the interstellar radiation field or the Lyman continuum flux from high mass O/B stars [49]. We note that the photoionization of H2 can lead on to the formation of H3+, which may be observable if its destruction due to dissociate recombination (reaction (3.8) in §§3b) is inefficient. This occurs if the reactions (3.6) and (3.7) dominate over reaction (3.8) or if the electrons are removed quickly enough, so that reaction (3.8) cannot occur. Rodríguez-Barrera et al. [49] have shown that the high atmosphere regions, where Lyman continuum radiation is effective, already contain a small fraction of small cloud particles. Electron attachment onto these cloud particles might decrease the efficiency of reaction (3.8) such that H3+ remains in the gas phase. The same may be achieved by magnetically coupling an electron to a brown dwarf's strong magnetic field of 1000–6000 G [50]. The magnetic field strength required to assure magnetic coupling of an electron is mainly dependent on the density of the collisional partners, _n_gas, and the electron temperature, _T_e, such that _B_e∼_n_gas(_m_e_T_e)1/2 [51]. Hence, this threshold decreases with the decreasing gas density in the upper atmosphere where H3+ forms as the result of photoionization of H2. Such localized interaction of an ionospheric environment with a local magnetic field has been traced through H3+ emission on Jupiter [52]. Lenz et al. [37] reported a non-detection of H3+ for the hot-Jupiter HD 209458b in secondary eclipse for spectroscopic observation of the planet's dayside. In the light of the above discussion, this implies that (i) the magnetic field is too weak to enable a sufficient electron acceleration to enable H2 dissociation on HD 209458b, (ii) that the local supply of electrons is too low for enabling enough collisions with H2 to occur (e.g. due to atmosphere being shielded by the host star from interstellar radiation field, atmosphere has not developed an ionosphere and remains at its hottest LTE dayside temperature of 1800 K (see [18,42])), and (iii) a high H2O or CO abundance destroys H3+ kinetically. We address the possibility of H3+ formation in brown dwarfs with our kinetic modelling approach in §3.
(c). Lightning on exoplanets and brown dwarfs
Brown dwarfs and many extrasolar planets will form clouds in their atmospheres. The cloud particles charge due to photochemical processes nearer the top of the cloud (similar to what was described in §§2b) and by tribolectric processes due to turbulence-driven particle–particle collisions [53]. The charge that a cloud particle acquires increases with the size of the particle. As cloud particles gravitationally settle into deeper layers of the atmospheres and bigger particles fall faster, a large-scale charge separation is established and an electrostatic potential difference can build up inside a cloud. The stored energy may become large enough to overcome the local breakdown potential such that a large-scale lightning inside these extraterestrial clouds emerges. The lightning discharge converts an initially semi-neutral gas of a moderate temperature of approximately 1000−2000 K into a plasma channel of approximately 30 000 K, which sends a shock wave into its immediate surrounding. While the discharge process will be relatively short-lived (of the order of seconds), the effect it has on the gas chemistry can prevail for longer.
Hodosán et al. [54,55] have investigated how much lightning would be required to reproduce a transient, unpolarized one-off radio signal of the exoplanet HAT-P-11b [56]. The parameters for one lightning strike were adopted from Saturn and from Earth. The caveats with these assumptions are that HAT-P-11b is a mini-Neptune with _M_P = 26 _M_Earth and _R_P = 4.7 _R_Earth [22], and all lightning measurements are carried out for Earth and detection for Solar System planets only. Using lightning strike statistics is, therefore, a formidable task, given that the Jupiter and Saturn measurements must be considered as incomplete (see discussion in [54]). While it is reasonable to assume that the electrostatic breakdown field does not vary strongly with the local composition of the gas (as demonstrated in [57]), the radiation power derived for HAT-P-11b at a distance of d = 38 pc from Earth for one lightning flash of 2.2 1014 Jy is based on values for a Saturnian lightning strike. The best case scenario would require 1015 of such Saturnian lightning strikes to produce an observation signal in radio frequency of ≈ 4 mJy during an observation time ≈ 40 min. This translates to a lightning flash density of 114 flashes km−2 h−1. The parameter study in Hodosán et al. [54] provides insight into the challenges involved. Such Saturnian lightning on the mini-Neptune HAT-P-11b would produce 2–8 times more radio power than terrestrial lightning but the time scales involved are rather short. Chemical tracers are therefore another option to trace the effect of lightning in planetary atmospheres. HCN is one candidate tracer because once created by the ion-neutral chemistry associated with lightning, it can be mixed up into atmospheric layers that may be observationally accessible. HCN would survive for 2–3 years in a planetary atmosphere with a moderate effective vertical mixing.
3. The presence of H3+ and H3O+ on brown dwarfs
It is likely that brown dwarfs have aurorae because brown dwarfs possess (strong) confining (magnetic) fields, free charges in the form of electrons and their collisional partners in the form of the atmospheric gas. Cyclotron maser emission (CME) has been detected from several brown dwarfs [58], which are produced by electron beams [59], plausibly from energetic electrons transported through the atmosphere along magnetic field lines [60], colliding with and ionizing the neutral species they encounter, just as auroral electrons ionize atmospheric species on Jupiter and Saturn [61]. Saturn and Jupiter receive their energetic electrons causing their auroral emission from their moons, Io and Europa, and Mercury receives its energetic electrons as part of the solar wind. Brown dwarfs cannot be argued to possess moons easily as they form like stars by gravitational collapse, and not like planets by collisions of boulders of different sizes in a protoplanetary disk. The energetic electrons required for an aurora must therefore come from the brown dwarf's atmosphere. Brown dwarfs can be expected to form an ionosphere in their outermost regions as a result of external irradiation as summarized in §§2b. Optical auroral emission has been observed on LSR-J1835 [29], an ultracool star hugging the stellar versus substellar boundary. We use LSR-J1835 as a representative case in what follows, modelling its atmospheric chemistry in the presence of UV irradiation from the interstellar medium, galactic cosmic rays and ionization via auroral electrons, each of which contribute to the formation of ions, principally H3+ and H3O+.
(a). The STAND2019 chemical network and ARGO high-energy chemistry model
Ion abundances deep in the atmospheres of brown dwarfs are close to chemical equilibrium [62–64]. If there were no UV photons, no energetic particles and no intrinsic disequilibrium processes active in the brown dwarf atmosphere, the results will not depart far from equilibrium except in the exosphere. Three body recombination deep in the atmosphere, dissociative recombination, and ion-neutral reactions, are fast (approx. 10−9 cm3 s−1 rate constants for most ion-neutral two-body reactions), and effectively barrier-less. Ions should not be quenched by vertical mixing.
The production of ions in the upper atmosphere of brown dwarfs is dominated by disequilibrium processes, such as photochemistry and energetic particle chemistry. To predict the effect of these processes on mixing ratios throughout the brown dwarf atmosphere, we solve the one-dimensional diffusion-energetic chemistry equation:
where n i [cm−3] is the number density of species i, t [s] is time, P i is the rate of production of that species and L i [cm−3 s−1] is the loss rate. The term ∂Φ i/d_z_ [cm−3 s−1] describes the vertical diffusion. We employ, ARGO, a Lagrangian chemical kinetics model [64], which is solved in the frame following a parcel of gas through the atmosphere, where:
Below the homopause, ∂Φ i/d_z_ ≈ ∂Φ_0/d_z, and so there:
Equation (3.2) is folded into the production and loss terms, which change with time dependent on the parcel's location in the atmosphere and the local temperature, pressure, UV and particle fluxes, as described by Rimmer & Helling [64]. Once the parcel returns to the base of the atmosphere, transport of UV photons and energetic particles is calculated, and depth-dependent ionization rates are determined. The chemistry is solved again using these rates, after which the photon and energetic particle transport is calculated again. These two calculations are iterated until the code converges on a solution.
ARGO employs the STAND2019 chemical network, which arises from the STAND2016 network [64], with updates to rate coefficients and the modification and addition of several reactions to better represent experimental results and observations of the Earth's atmosphere [65]. STAND2019 includes over 5000 reactions incorporating H/C/N/O, complete for two carbon species, two nitrogen species, and three oxygen species, and valid for temperatures between 200 and 30 000 K. It has been benchmarked against the modern Earth, Jupiter [64] and hot Jupiter models [66,67], and has been applied to ultra-hot Jupiters [68,69], early Earth atmospheres [70], lightning in exoplanet atmospheres [55,71], and terrestrial magma chemistry [72].
We apply this chemical network to a PHOENIX model M 8.5 dwarf atmosphere [73,74] used to represent LSR-J1835. This model atmosphere has an effective temperature of T_eff = 2600 K, surface gravity of log_g = 5 and the mean molecular mass of 2.33 amu. ARGO also needs a time scale for vertical mixing. We can estimate this time scale, t z [s] and Eddy diffusion coefficient, K zz [cm2 s−1] from the convective velocity, _v_conv [cm s−1], one of the PHOENIX model outputs, using equations (6) and (7) from Lee et al. [42]:
and
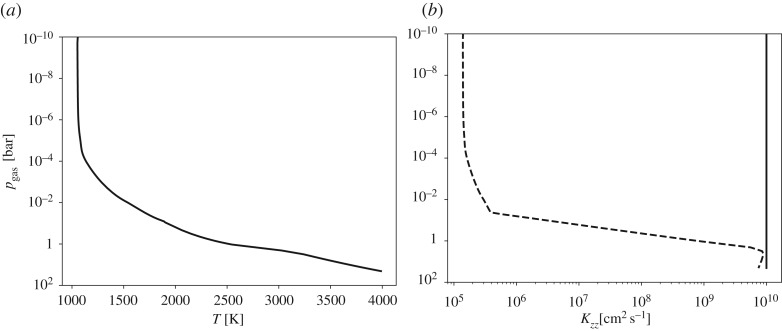
where _H_0 [cm] is the atmospheric scale height. Eddy diffusion is driven not simply by bulk convection but by the turbulent motion within convective cells, and may be much higher. For our calculations, we use a constant K zz = 1010 cm2 s−1, but we will use both the estimate from equation (3.4) and this constant value when comparing with the chemical time scales below. The temperature profile and Eddy diffusion coefficients are shown in figure 3.
Figure 3.
(a) Temperature [K] and (b) eddy diffusion K zz [cm2 s−1] as a function of pressure for a PHOENIX model M 8.5 dwarf, with an effective temperature of T_eff = 2600 K, surface gravity of log_g = 5 and the mean molecular mass of 2.33 amu.
(b). Photochemical generation of H3+ on brown dwarfs
Photoionization of H2 in a gas giant atmosphere, whether within (e.g. [30,31]), or outside our Solar System [34,75], will readily lead to the formation of H3+ via the reactions:
and
If H2 is the most probable second body, then virtually all of the H+2 will react with H2 to form H3+, and H3+ will be destroyed by either dissociative recombination with its electron:
The steady-state concentration of H2 will be dependent on actinic flux of UV photons (the number of photons per cm2 per second per Å integrated over a unit sphere), multiplied by the cross section for reaction (3.6). This cross section is very large (approx. 10−17 cm2) for energies above near the ionization energy for H2 ( ≳15.9 eV). This means that reaction (3.6) is very efficient, even for a relatively low actinic flux, but also that H2 will self-shield over a relatively short distance.
Figures 4 and 5 show that some H3+ is generated within the thermosphere and exosphere of a brown dwarf from interstellar UV irradiation alone. Interstellar cosmic rays also contribute to the generation of H3+ in the upper atmospheres of free-floating ultracool stars.
Figure 4.
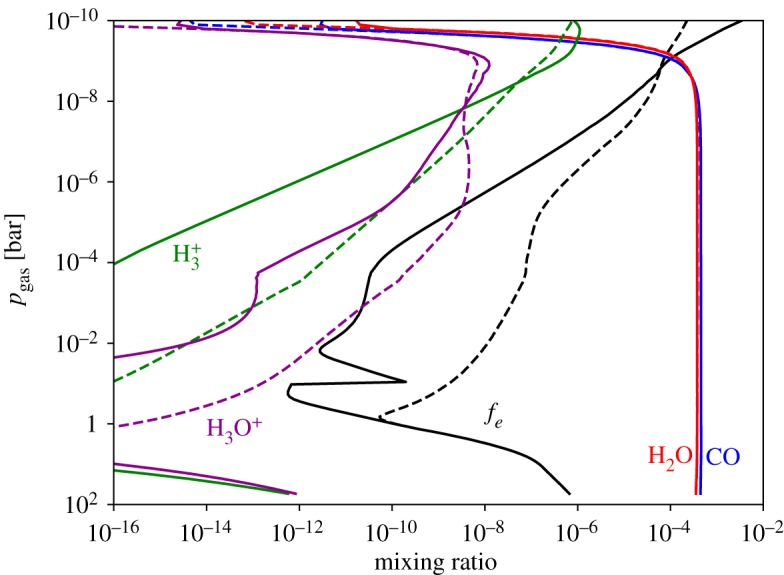
Mixing ratios versus pressure [_p_gas, bar] for the ions H3+ and H3O+, as well as the species involved in their destruction (e−, CO and H2O). Solid lines represent the results when accounting for photochemistry and cosmic ray chemistry, and dashed lines represent the results when including an auroral electron beam, as described in §§3d. (Online version in colour.)
Figure 5.
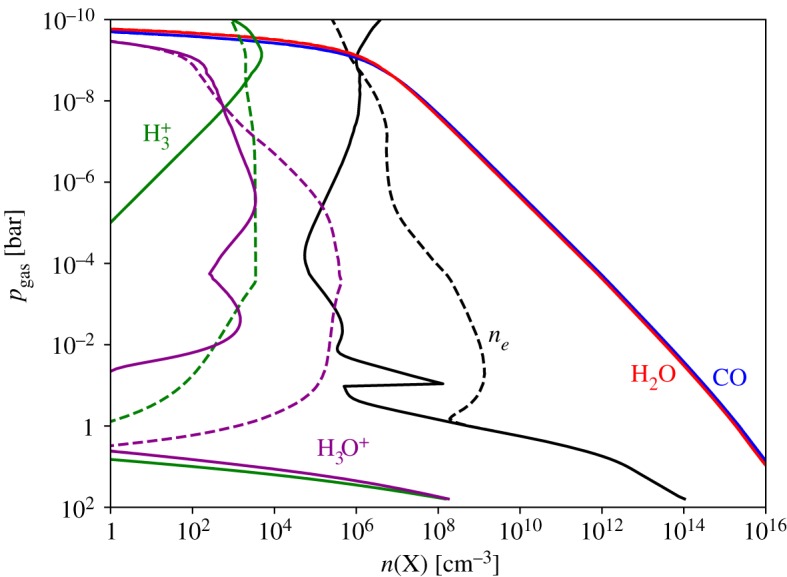
Gas-phase number density (n [cm−3]) versus pressure (_p_gas [bar]) for the ions H3+ and H3O+, as well as the species involved in their destruction (e−, CO and H2O). Solid lines represent the results when accounting for photochemistry and cosmic ray chemistry, and dashed lines represent the results when including an auroral electron beam, as described in §§3d. (Online version in colour.)
(c). Mechanism for generating H3+ by electron beam
Strong electron beams have been inferred in the atmospheres of some ultracool stars, such as LSR-J1835 [29]. The mechanism for generating H3+ from an electron beam is identical to the mechanism above, except for reaction (3.6), which is instead [34]:
| H2+e−,∗→H2++e−,∗+e−, | 3.10 |
|---|
where e−,* represents a high-energy electron. For the ionization cross section, σ i(E), we use the values given by Padovani et al. [76] and Rimmer & Helling [77]. This chemical mechanism for generating H3+ is similar to the mechanism discussed for hot Jupiters by Chadney et al. [78].
Although the chemistry is very similar between electron-induced ionization and photoionization, the physical process is different, and so the part of the atmosphere affected is also different. In order to model the effect of electron-induced ionization in a brown dwarf atmosphere, we need to consider the incident flux of electrons. We will use the aurora detected on the M 8.5 dwarf LSR-J1835 as a representative auroral electron beam impinging on brown dwarf atmospheres. As we discussed above, the intensity of the CME from LSR-J1835 is 105 times that of Jupiter. This can be explained either by shifting the electron energy up, or by increasing the number of electrons by 105. Shifting the electron energy too far will result in electron synchrotron emission, and will thus remove the CME. More intense CMEs are best explained by a greater number of keV electrons [79]. On this basis, and the observed CME intensity from LSR-J1835, we simply take the flux for the auroral electron beam for Jupiter [80], and multiply it by 105. For the Jovian auroral electron beam, we use the form of Gérard & Singh [80], where E [eV] is the electron energy, _j_0 = 1.25 × 1015 electrons cm−2 s−1 eV−1, and _E_0 [eV] is the characteristic electron energy. We use 5 keV for the characteristic electron energy, as inferred by Gérard et al. [81]:
and multiply it by 105, to yield:
This assumes that the energy of the electron beam scales with the overall auroral energy, but since the auroral electron energy is estimated from the CME observed on LSR-J1835, and the intensity of this emission depends on the number of electrons, this seems to be a reasonable approximation. We could use other methods to adapt a Jovian auroral electron beam to LSR-J1835, such as shifting the energy of the electrons instead of increasing their number, but we could only adopt this method to a limited extent, because CME requires non-relativistic electrons, and so limits us to electrons of energy ≲500 keV. As brown dwarfs are exposed to harsher external radiation fields leading to highly ionized upper atmospheres [49], it is most plausible that the difference in intensity of the aurora on LSR-J1835, compared with Jupiter, is due to a greater number of electrons.
In order to find how the energy-dependent flux of the electron beam evolves as the electron beam penetrates from the top into the atmosphere, we follow the same approach of [82], where we apply a Monte Carlo model with 100 000 test electrons, which are injected into the top of the atmosphere with initial energies, E i representative of the energy-dependent flux appropriate to the electron bream. At each step through the atmosphere, dz, each electron is assigned a random value distributed uniformly between [0,1], and this value is compared with the probability, P(E i) that an electron of energy E i [eV] would experience an inelastic collision:
where _n_gas [cm−3] is the gas-phase density, and σ(E i) [cm2] is the total cross section for an interaction. If the random number is less than P(E i), an interaction occurs, and a second random number, uniformly distributed between [0,1], is assigned to the electron, and this number is compared with the normalized cross section to dissociate (σ d), excite (σ _JJ_′) or ionize (σ i) the electron, where:
| ΣJ′σJJ′(E)+σd(E)+σi(E)=σ(E). | 3.13 |
|---|
Each of these interactions has a characteristic energy loss, W [eV], which is subtracted from the energy of the electron. The electrons are binned by energy after d_z_, and this is the updated spectrum of the electron beam. This is the same as the Monte Carlo model for cosmic ray energy loss presented by Rimmer et al. [83] and Rimmer & Helling [77] but for electrons instead of cosmic ray protons.
Auroral electrons penetrate much more deeply into brown dwarf atmospheres than UV photons, and as a result the H3+ profile extends much further into the brown dwarf atmosphere, as can be seen in figures 4 and 5. The H3+ concentration drops off precipitously approaching 1 bar, and this indicates the attenuation of the electron beam.
(d). Destruction of H3+ and the formation of H3O+ in brown dwarf atmospheres
In the upper atmosphere, H3+ is primarily destroyed by recombination with its electron. Because H3+ reacts rapidly with common constituents in brown dwarf atmospheres, CO and H2O, its chemical lifetime overall is short and density-dependent. The relevant reactions are:
| H3++e−→products,k1=2.8×10−8 cm3 s−1 (T300 K)−0.52; | 3.14 |
|---|
| H3++CO→HCO++H2,k2=1.4×10−9 cm3 s−1; | 3.15 |
|---|
| andH3++H2O→H3O++H2k3=4.3×10−9 cm3 s−1. | 3.16 |
|---|
The ion HCO+ reacts very quickly with other atmospheric constituents and its abundance is very low, with mixing ratios less than 10−16 throughout. Hydronium (H3O+), on the other hand, is more stable, and becomes the dominant hydrogen-bearing ion in the brown dwarf's lower atmosphere (figures 4 and 5). The H3O+ is destroyed by electrons and ammonia, with the reactions:
| H3O++e−→products,k4=4.3×10−7 cm3 s−1 (T300 K)−0.5; | 3.17 |
|---|
| andH3O++NH3→NH4++H2O,k5=2.5×10−9 cm3 s−1. | 3.18 |
|---|
Since these are the dominant destruction pathways for H3+ and H3O+, and the products do not cycle back to reform H3+ and H3O+, the above rate constants can be used directly to estimate the chemical lifetimes of these cations.
For H3+, the chemical lifetime is:
| τchem(H3+)=[H3+]d[H3+]/dt=1k1[e−1]+k2[CO]+k3[H2O], | 3.19 |
|---|
and for H3O+:
| τchem(H3O+)=[H3O+]d[H3O+]/dt=1k4[e−1]+k5[NH3]. | 3.20 |
|---|
The chemical time scales for H3+ and H3O+ are shown in figure 6. These time scales can be compared with dynamic time scales, _t_dyn, estimated using vertical or horizontal mixing velocities. When _t_dyn > _t_chem, the chemistry is driven out of equilibrium by photodissociation and photoionization, but is not much influenced by the dynamics of the fluid motion in the atmosphere. When _t_dyn > _t_chem, on the other hand, the dynamics dominates, and the species can be transported into regions with concentrations far from equilibrium. If we compare the chemical time scale to the dynamical time scale calculated using equation (3.4), we find the dynamical time scale is orders of magnitude larger than the chemical time scale throughout the brown dwarf atmosphere, and the ion chemistry is never quenched. On the other hand, if we compare the chemical time scale with the time scale derived from our constant K zz = 1010 cm2 s−1 (as in figure 6), we find that the chemical time scale exceeds the dynamical time scale in the upper atmosphere, and so the ion chemistry may be quenched in the upper atmosphere with efficient vertical mixing.
Figure 6.
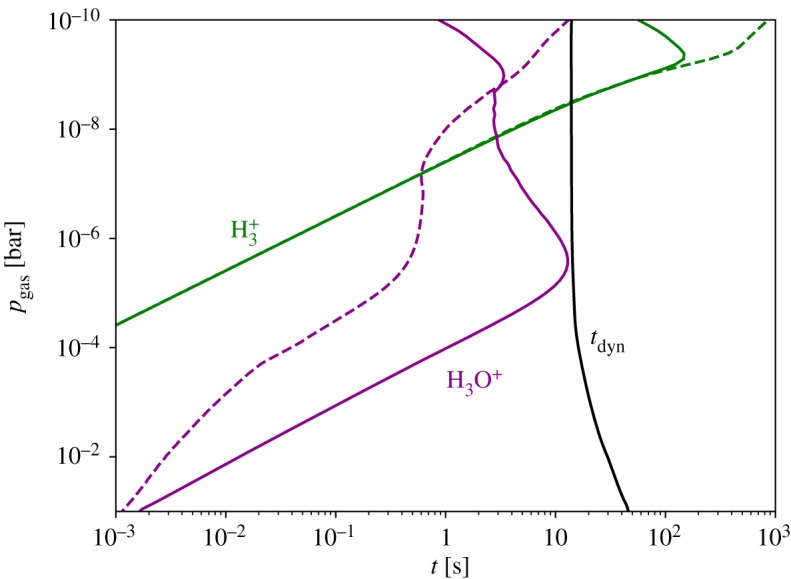
Chemical time scales for H3+ and H3O+, ranging from ≲1 ms where _p_gas > 10−1 bar to 10–100 s where _p_gas < 10−4 bar. Solid lines represent the results when accounting for photochemistry and cosmic ray chemistry, and dashed lines represent the results when including an auroral electron beam, as described in §§3d. The black solid line represents the dynamical time scale, _t_dyn [s], corresponding to a constant K zz = 1010 cm2 s−1. (Online version in colour.)
4. Conclusion
The atmospheres of brown dwarfs and hot Jupiters are witness to physical and chemical processes in regimes that can be very different from anything in our Solar System. Atmospheric temperatures range between <300…∼3500 K for irradiated planets, and the contrast between day and night can be extreme, resulting in planet-scale equatorial jets that respond to the steep temperature gradients. Cloud particles form from seed particles made of, for example, titanium dioxide and silicon monoxide, which grow a mantle containing a mix of Mg/Si/Fe/Al/Ti/C/…/O minerals, fall through the atmosphere like rain, until they are dissolved deep in the atmosphere by temperatures high enough to sublimate iron. For all these extremes, hot Jupiters and brown dwarfs share some surprising similarities with the gas giants in our Solar System. The particles (aerosols) that make up their clouds can become ionized, and the electrification and subsequent advection of cloud particles generates charge separation over atmospheric scales, and may lead to lightning. Aurorae on brown dwarfs, though 105 times more intense, and although probably not generated by a moon or rings, are explained by the same fundamental mechanisms that generate aurorae on Jupiter and Saturn. The source of the electrons, the location of the electron-gas interaction, and the relative abundances of the gas species are different but the immediate chemical products of the electron-gas interactions, H+2 and H3+, are the same within these highly diverse, but hydrogen-dominated atmospheres.
We find that the chemistry that leads to H3+ generation on hot Jupiters and brown dwarfs is the same as the chemistry on Jupiter and Saturn. The gas giants in our Solar System are very cold, so that water freezes out much deeper in their atmospheres than for extrasolar hot Jupiters and warm brown dwarfs. Therefore, less H2O and CO remains in the gas phase compared with hot Jupiters, L-type brown dwarfs and very late M-type dwarfs. Consequently, when present, the water vapour and carbon monoxide react readily with H3+ to form HCO+ and hydronium (H3O+), respectively. Interstellar UV radiation and galactic cosmic rays generate H3+ at microbar to nanobar pressures, and H3O+ at millibar to microbar pressures. The energetic electrons that would drive aurorae on brown dwarfs ionize hydrogen much deeper in their atmosphere, generating 106 cm−3 densities of H3O+ at 1 bar.
Producing H3+ requires molecular hydrogen, and this may be one of the reasons H3+ has proved difficult to detect in hot Jupiter atmospheres. The irradiation would ionize H2, but also dissociates H2. The 10 000 K thermospheric temperatures expected for Hot Jupiters [84], along with the intense radiation, means that the most prevalent neutral species at these heights is atomic hydrogen, and the most abundant ion is H+. Since f e/f(_H_3+) > 1 in the upper atmospheres of Hot Jupiters, it will be difficult to sustain much H3+, as has already been shown by Chadney et al. [78]. In the deeper, radiation-sheltered atmosphere, H3+ will again be destroyed by collisions with H2O and CO similar to brown dwarfs. Brown dwarfs, on the other hand, may have much cooler upper atmospheres, amenable to the stability of H2, and therefore the efficient production of H3+, either via galactic cosmic rays and interstellar UV irradiation, or by collisional ionization with the high-energy electrons that would generate brown dwarf aurorae. The lifetime of H3+ is very short in the deeper atmosphere, primarily because it reacts quickly with CO and H2O. The reaction of H3+ with H2O results in H3O+. We propose searching for H3+ and H3O+ in free-floating brown dwarfs. For robust identification, absorption cross sections valid for the extreme temperatures of these sub-stellar objects are needed. The ExoMol team already provides the needed H3+ line-lists [85], but updated line-lists for H3O+ will be needed if this molecule is to be positively identified in a brown dwarf atmosphere.
Acknowledgments
We thank Jonathan Tennyson, Steve Miller and Benjamin McCall for organizing an engaging Royal Society discussion meeting issue ‘Advances in Hydrogen Molecular Ions: H3+, H5+ and beyond’. We thank Sergey Yurchenko for useful discussions about high-temperature ion cross sections. We thank Piere Gourbin for providing figure 1 and V. Parmentier for providing the cloud-free three-dimensional GCM results for WASP-18b.
Data accessibility
This article does not contain any additional data.
Author's contributions
Ch.H. led the paper, and carried out the cloud and equilibrium gas phase calculations. P.B.R. performed the chemical kinetics calculations, and created figures 3–6. Both authors contributed equally to writing the paper.
Competing interests
We declare we have no competing interests.
Funding
P.B.R. thanks the Simons Foundation for funding (SCOL awards no. 599634). Ch.H. and P.B.R. acknowledge funding from the European Commission, under which part of this research was conducted. We highlight financial support from the European Union under the FP7 by an ERC starting grant no. 257431.
References
- 1.Batalha NM. 2014. Exploring exoplanet populations with NASA's Kepler Mission. Proc. Natl Acad. Sci. USA 111, 12 647–12 654. ( 10.1073/pnas.1304196111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tinetti G._et al._2007. Water vapour in the atmosphere of a transiting extrasolar planet. Nature 448, 169–171. ( 10.1038/nature06002) [DOI] [PubMed] [Google Scholar]
- 3.Désert JM, Lecavelier-des-Etangs A, Hébrard G, Sing DK, Ehrenreich D, Ferlet R, Vidal-Madjar A. 2009. Search for carbon monoxide in the atmosphere of the transiting exoplanet HD 189733b. Astrophys. J. 699, 478–485. ( 10.1088/0004-637X/699/1/478) [DOI] [Google Scholar]
- 4.Kreidberg L._et al._2018. Global climate and atmospheric composition of the ultra-hot Jupiter WASP-103b from HST and spitzer phase curve observations. Astron. J. 156, 17 ( 10.3847/1538-3881/aac3df) [DOI] [Google Scholar]
- 5.Nikolov N._et al._2018. An absolute sodium abundance for a cloud-free ‘hot Saturn’ exoplanet. Nature 557, 526–529. ( 10.1038/s41586-018-0101-7) [DOI] [PubMed] [Google Scholar]
- 6.Brogi M, Giacobbe P, Guilluy G, de Kok RJ, Sozzetti A, Mancini L, Bonomo AS. 2018. Exoplanet atmospheres with GIANO. I. Water in the transmission spectrum of HD 189733b. Astron. Astrophys. 615, A16 ( 10.1051/0004-6361/201732189) [DOI] [Google Scholar]
- 7.Sing DK._et al._2011. Hubble space telescope transmission spectroscopy of the exoplanet HD 189733b: high-altitude atmospheric haze in the optical and near-ultraviolet with STIS. Mon. Not. R. Astron. Soc. 416, 1443–1455. ( 10.1111/j.1365-2966.2011.19142.x) [DOI] [Google Scholar]
- 8.Louden T, Wheatley PJ. 2015. Spatially resolved eastward winds and rotation of HD 189733b. Astrophys. J. 814, L24 ( 10.1088/2041-8205/814/2/L24) [DOI] [Google Scholar]
- 9.Knutson HA, Charbonneau D, Allen LE, Fortney JJ, Agol E, Cowan NB, Showman AP, Cooper CS, Megeath ST. 2007. A map of the day-night contrast of the extrasolar planet HD 189733b. Nature 447, 183–186. ( 10.1038/nature05782) [DOI] [PubMed] [Google Scholar]
- 10.Gelino CR, Marley MS, Holtzman JA, Ackerman AS, Lodders K. 2002. L dwarf variability: I-band observations. Astrophys. J. 577, 433–446. ( 10.1086/apj.2002.577.issue-1) [DOI] [Google Scholar]
- 11.Radigan J, Jayawardhana R, Lafrenière D, Artigau É, Marley M, Saumon D. 2012. Large-amplitude variations of an L/T transition brown dwarf: multi-wavelength observations of patchy, high-contrast cloud features. Astrophys. J. 750, 105 ( 10.1088/0004-637X/750/2/105) [DOI] [Google Scholar]
- 12.Apai D, Radigan J, Buenzli E, Burrows A, Reid IN, Jayawardhana R. 2013. HST spectral mapping of L/T transition brown dwarfs reveals cloud thickness variations. Astrophys. J. 768, 121 ( 10.1088/0004-637X/768/2/121) [DOI] [Google Scholar]
- 13.Vos JM._et al._2019. A search for variability in exoplanet analogues and low-gravity brown dwarfs. Mon. Not. R. Astron. Soc. 483, 480–502. [Google Scholar]
- 14.Carone L, Keppens R, Decin L. 2014. Connecting the dots: a versatile model for the atmospheres of tidally locked Super-Earths. Mon. Not. R. Astron. Soc. 445, 930–945. ( 10.1093/mnras/stu1793) [DOI] [Google Scholar]
- 15.Marley MS, Saumon D, Cushing M, Ackerman AS, Fortney JJ, Freedman R. 2012. Masses, radii, and cloud properties of the HR 8799 planets. Astrophys. J. 754, 135 ( 10.1088/0004-637X/754/2/135) [DOI] [Google Scholar]
- 16.Lee G, Dobbs-Dixon I, Helling C, Bognar K, Woitke P. 2016. Dynamic mineral clouds on HD 189733b. I. 3D RHD with kinetic, non-equilibrium cloud formation. Astron. Astrophys. 594, A48 ( 10.1051/0004-6361/201628606) [DOI] [Google Scholar]
- 17.Zhang X, Showman AP. 2017. Effects of bulk composition on the atmospheric dynamics on close-in exoplanets. Astrophys. J. 836, 73 ( 10.3847/1538-4357/836/1/73) [DOI] [Google Scholar]
- 18.Lines S._et al._2018. Simulating the cloudy atmospheres of HD 209458 b and HD 189733 b with the 3D Met Office unified model. Astron. Astrophys. 615, A97 ( 10.1051/0004-6361/201732278) [DOI] [Google Scholar]
- 19.Helling C. 2018. Exoplanet clouds. (https://arxiv.org/abs/1812.03793).
- 20.Füllekrug M._et al._2013. Energetic charged particles above thunderclouds. Surv. Geophys. 34, 1–41. ( 10.1007/s10712-012-9205-z) [DOI] [Google Scholar]
- 21.Füllekrug M._et al._2013. Electron acceleration above thunderclouds. Environ. Res. Lett. 8, 035027 ( 10.1088/1748-9326/8/3/035027) [DOI] [Google Scholar]
- 22.Hodosán G, Helling C, Asensio-Torres R, Vorgul I, Rimmer PB. 2016. Lightning climatology of exoplanets and brown dwarfs guided by Solar System data. Mon. Not. R. Astron. Soc. 461, 3927–3947. ( 10.1093/mnras/stw1571) [DOI] [Google Scholar]
- 23.Helling C, Rimmer PB, Rodriguez-Barrera IM, Wood K, Robertson GB, Stark CR. 2016. Ionisation and discharge in cloud-forming atmospheres of brown dwarfs and extrasolar planets. Plasma Phys. Control. Fusion 58, 074003 ( 10.1088/0741-3335/58/7/074003) [DOI] [Google Scholar]
- 24.Desch SJ, Cuzzi JN. 2000. The generation of lightning in the solar nebula. Icarus 143, 87–105. ( 10.1006/icar.1999.6245) [DOI] [Google Scholar]
- 25.Helling C._et al._2016. Atmospheric electrification in dusty, reactive gases in the solar system and beyond. Surv. Geophys. 37, 705–756. ( 10.1007/s10712-016-9361-7) [DOI] [Google Scholar]
- 26.Longstaff ES, Casewell SL, Wynn GA, Maxted PFL, Helling C. 2017. Emission lines in the atmosphere of the irradiated brown dwarf WD0137-349B. Mon. Not. R. Astron. Soc. 471, 1728–1736. ( 10.1093/mnras/stx1786) [DOI] [Google Scholar]
- 27.Casewell SL, Littlefair SP, Parsons SG, Marsh TR, Fortney JJ, Marley MS. 2018. The direct detection of the irradiated brown dwarf in the white dwarf-brown dwarf binary SDSS J141126.20+200911.1. Mon. Not. R. Astron. Soc. 481, 5216–5222. ( 10.1093/mnras/sty2599) [DOI] [Google Scholar]
- 28.Berger E. 2002. Flaring up all over-radio activity in rapidly rotating late M and L Dwarfs. Astrophys. J. 572, 503–513. ( 10.1086/apj.2002.572.issue-1) [DOI] [Google Scholar]
- 29.Hallinan G._et al._2015. Magnetospherically driven optical and radio aurorae at the end of the stellar main sequence. Nature 523, 568–571. ( 10.1038/nature14619) [DOI] [PubMed] [Google Scholar]
- 30.Gross SH, Rasool SI. 1964. The upper atmosphere of jupiter. Icarus 3, 311–322. ( 10.1016/0019-1035(64)90040-5) [DOI] [Google Scholar]
- 31.Atreya SK, Donahue TM. 1976. Model ionospheres of Jupiter. In IAU Colloq. 30: Jupiter: Studies of the Interior, Atmosp here, Magnetosphere and Satellites (eds. T Gehrels, S Matthews), pp. 304–318.
- 32.Hamilton DC, Gloeckler G, Krimigis SM, Bostrom CO, Armstrong TP, Axford WI, Fan CY, Lanzerotti LJ, Hunten DM. 1980. Detection of energetic hydrogen molecules in Jupiter's magnetosphere by Voyager 2 - Evidence for an ionospheric plasma source. Geophys. Res. Lett. 7, 813–816. ( 10.1029/GL007i010p00813) [DOI] [Google Scholar]
- 33.Drossart P._et al._1989. Detection of H3+ on Jupiter. Nature 340, 539–541. ( 10.1038/340539a0) [DOI] [Google Scholar]
- 34.Miller S._et al._2000. The role of H3+ in planetary atmospheres. Phil. Trans. R. Soc. Lond. A 358, 2359–2559. ( 10.1098/rsta.2000.0662) [DOI] [Google Scholar]
- 35.Goto M, Geballe TR, McCall BJ, Usuda T, Suto H, Terada H, Kobayashi N, Oka T. 2005. Search for H3+ in HD 141569A. Astrophys. J. 629, 865–872. ( 10.1086/apj.2005.629.issue-2) [DOI] [Google Scholar]
- 36.Shkolnik E, Gaidos E, Moskovitz N. 2006. No detectable H3+ emission from the atmospheres of hot jupiters. Astron. J. 132, 1267–1274. ( 10.1086/506476) [DOI] [Google Scholar]
- 37.Lenz LF, Reiners A, Seifahrt A, Käufl HU. 2016. A CRIRES-search for H3+ emission from the hot Jupiter atmosphere of HD 209458b. Astron. Astrophys. 589, A99 ( 10.1051/0004-6361/201525675) [DOI] [Google Scholar]
- 38.Casewell SL._et al._2015. Multiwaveband photometry of the irradiated brown dwarf WD0137-349B. Mon. Not. R. Astron. Soc. 447, 3218–3226. ( 10.1093/mnras/stu2721) [DOI] [Google Scholar]
- 39.Helling C, Gourbin P, Woitke P, Parmentier V. 2019 Sparkling nights and very hot days on WASP-18b: the formation of clouds and the emergence of an ionosphere. (https://arxiv.org/abs/1901.08640. )
- 40.Parmentier V._et al._2018. From thermal dissociation to condensation in the atmospheres of ultra hot Jupiters: WASP-121b in context. Astron. Astrophys. 617, A110 ( 10.1051/0004-6361/201833059) [DOI] [Google Scholar]
- 41.Helling C._et al._2016. The mineral clouds on HD 209458b and HD 189733b. Mon. Not. R. Astron. Soc. 460, 855–883. ( 10.1093/mnras/stw662) [DOI] [Google Scholar]
- 42.Lee G, Helling C, Dobbs-Dixon I, Juncher D. 2015. Modelling the local and global cloud formation on HD 189733b. Astron. Astrophys. 580, A12 ( 10.1051/0004-6361/201525982) [DOI] [Google Scholar]
- 43.Jørgensen UG. 1997. Cool star models. In IAU Symposium (ed. EF van Dishoeck), vol. 178, pp. 441–456.
- 44.Tsuji T, Ohnaka K, Aoki W, Nakajima T. 1996. Evolution of dusty photospheres through red to brown dwarfs: how dust forms in very low mass objects. Astron. Astrophys. 308, L29–L32. [Google Scholar]
- 45.Allard F, Hauschildt PH, Alexander DR, Starrfield S. 1997. Model atmospheres of very low mass stars and brown dwarfs. Annu. Rev. Astron. Astrophys. 35, 137–177. ( 10.1146/annurev.astro.35.1.137) [DOI] [Google Scholar]
- 46.Charnay B, Bézard B, Baudino JL, Bonnefoy M, Boccaletti A, Galicher R. 2018. A self-consistent cloud model for brown dwarfs and young giant exoplanets: comparison with photometric and spectroscopic observations. Astrophys. J. 854, 172 ( 10.3847/1538-4357/aaac7d) [DOI] [Google Scholar]
- 47.Dobbs-Dixon I, Agol E. 2013. Three-dimensional radiative-hydrodynamical simulations of the highly irradiated short-period exoplanet HD 189733b. Mon. Not. R. Astron. Soc. 435, 3159–3168. ( 10.1093/mnras/stt1509) [DOI] [Google Scholar]
- 48.Showman AP, Tan X, Zhang X. 2018 Atmospheric circulation of brown dwarfs and Jupiter and Saturn-like planets: zonal jets, long-term variability, and QBO-type oscillations. (https://arxiv.org/abs/1807.08433. )
- 49.Rodríguez-Barrera MI, Helling C, Wood K. 2018. Environmental effects on the ionisation of brown dwarf atmospheres. Astron. Astrophys. 618, A107 ( 10.1051/0004-6361/201832685) [DOI] [Google Scholar]
- 50.Kao MM, Hallinan G, Pineda JS, Stevenson D, Burgasser A. 2018. The strongest magnetic fields on the coolest brown dwarfs. ApJS 237, 25 ( 10.3847/1538-4365/aac2d5) [DOI] [Google Scholar]
- 51.Rodríguez-Barrera MI, Helling C, Stark CR, Rice AM. 2015. Reference study to characterize plasma and magnetic properties of ultracool atmospheres. Mon. Not. R. Astron. Soc. 454, 3977–3995. ( 10.1093/mnras/stv2090) [DOI] [Google Scholar]
- 52.Stallard TS._et al._2018. Identification of Jupiter's magnetic equator through H3+ ionospheric emission. Nat. Astron. 2, 773–777. ( 10.1038/s41550-018-0523-z) [DOI] [Google Scholar]
- 53.Helling C, Jardine M, Mokler F. 2011. Ionization in atmospheres of brown dwarfs and extrasolar planets. II. Dust-induced collisional ionization. Astrophys. J. 737, 38 ( 10.1088/0004-637X/737/1/38) [DOI] [Google Scholar]
- 54.Hodosán G, Rimmer PB, Helling C. 2016. Is lightning a possible source of the radio emission on HAT-P-11b? Mon. Not. R. Astron. Soc. 461, 1222–1226. ( 10.1093/mnras/stw977) [DOI] [Google Scholar]
- 55.Hodosán G, Helling C, Rimmer PB. 2017. Exo-lightning radio emission: the case study of HAT-P-11b. Planet. Radio Emiss. VIII 345–356. (https://arxiv.org/abs/1711.08053) [Google Scholar]
- 56.Lecavelier des Etangs A, Sirothia SK, Gopal-Krishna, Zarka P. 2013. Hint of 150 MHz radio emission from the Neptune-mass extrasolar transiting planet HAT-P-11b. Astron. Astrophys. 552, A65 ( 10.1051/0004-6361/201219789) [DOI] [Google Scholar]
- 57.Helling C, Jardine M, Stark C, Diver D. 2013. Ionization in atmospheres of brown dwarfs and extrasolar planets. III. Breakdown conditions for mineral clouds. Astrophys. J. 767, 136 ( 10.1088/0004-637X/767/2/136) [DOI] [Google Scholar]
- 58.Hallinan G, Antonova A, Doyle JG, Bourke S, Lane C, Golden A. 2008. Confirmation of the electron cyclotron maser instability as the dominant source of radio emission from very low mass stars and brown dwarfs. Astrophys. J. 684, 644–653. ( 10.1086/529168) [DOI] [Google Scholar]
- 59.Schneider J. 1959. Stimulated emission of radiation by relativistic electrons in a magnetic field. Phys. Rev. Lett. 2, 504–505. ( 10.1103/PhysRevLett.2.504) [DOI] [Google Scholar]
- 60.Nichols JD, Burleigh MR, Casewell SL, Cowley SWH, Wynn GA, Clarke JT, West AA. 2012. Origin of electron cyclotron maser induced radio emissions at ultracool dwarfs: magnetosphere-ionosphere coupling currents. Astrophys. J. 760, 59 ( 10.1088/0004-637X/760/1/59) [DOI] [Google Scholar]
- 61.Grodent D. 2015. A brief review of ultraviolet auroral emissions on giant planets. Space Sci. Rev. 187, 23–50. ( 10.1007/s11214-014-0052-8) [DOI] [Google Scholar]
- 62.Lavvas P, Koskinen T, Yelle RV. 2014. Electron densities and alkali atoms in exoplanet atmospheres. Astrophys. J. 796, 15 ( 10.1088/0004-637X/796/1/15) [DOI] [Google Scholar]
- 63.Rimmer PB, Helling C, Bilger C. 2014. The influence of galactic cosmic rays on ion-neutral hydrocarbon chemistry in the upper atmospheres of free-floating exoplanets. Int. J. Astrobiol. 13, 173–181. ( 10.1017/S1473550413000487) [DOI] [Google Scholar]
- 64.Rimmer PB, Helling C. 2016. A chemical kinetics network for lightning and life in planetary atmospheres. Astrophys. J. Suppl. Ser. 224, 9 ( 10.3847/0067-0049/224/1/9) [DOI] [Google Scholar]
- 65.Rimmer PB, Rugheimer S. 2019. Hydrogen cyanide in nitrogen-rich atmospheres of rocky exoplanets. Icarus 329, 124–131. ( 10.1016/j.icarus.2019.02.020) [DOI] [Google Scholar]
- 66.Tsai SM, Lyons JR, Grosheintz L, Rimmer PB, Kitzmann D, Heng K. 2017. VULCAN: an open-source, validated chemical kinetics python code for exoplanetary atmospheres. Astrophys. J. Suppl. Ser. 228, 20 ( 10.3847/1538-4365/228/2/20) [DOI] [Google Scholar]
- 67.Hobbs R, Shorttle O, Madhusudhan N, Rimmer PB. 2019. A chemical kinetics code for modelling exoplanet atmospheres. Mon. Not. R. Astron. Soc. 487, 2242–2261. ( 10.1093/mnras/stz1333) [DOI] [Google Scholar]
- 68.Kitzmann D._et al._2018. The peculiar atmospheric chemistry of KELT-9b. Astrophys. J. 863, 183 ( 10.3847/1538-4357/aace5a) [DOI] [Google Scholar]
- 69.Hoeijmakers HJ._et al._2018. Atomic iron and titanium in the atmosphere of the exoplanet KELT-9b. Nature 560, 453–455. ( 10.1038/s41586-018-0401-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rimmer PB, Shorttle O, Rugheimer S. 2019. Oxidised micrometeorites as evidence for low atmospheric pressure on the early Earth. Geochem. Perspect. Lett. 9, 38–42. ( 10.7185/geochemlet.1903) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ardaseva A, Rimmer PB, Waldmann I, Rocchetto M, Yurchenko SN, Helling C, Tennyson J. 2017. Lightning chemistry on Earth-like exoplanets. Mon. Not. R. Astron. Soc. 470, 187–196. ( 10.1093/mnras/stx1012) [DOI] [Google Scholar]
- 72.Rimmer PB, Shorttle O. 2019. Origin of life's building blocks in carbon- and nitrogen-rich surface hydrothermal vents. Life 9, 12 ( 10.3390/life9010012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Allard F, Hauschildt PH, Schweitzer A. 2000. Spherically symmetric model atmospheres for low-mass pre-main-sequence stars with effective temperatures between 2000 and 6800 K. Astrophys. J. 539, 366–371. ( 10.1086/apj.2000.539.issue-1) [DOI] [Google Scholar]
- 74.Allard F, Homeier D, Freytag B. 2012. Models of very-low-mass stars, brown dwarfs and exoplanets. Phil. Trans. R. Soc. Lond. A 370, 2765–2777. ( 10.1098/rsta.2011.0269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miller S, Stallard T, Tennyson J, Melin H. 2013. Cooling by H3+ emission. J. Phys. Chem. A 117, 9770–9777. ( 10.1021/jp312468b) [DOI] [PubMed] [Google Scholar]
- 76.Padovani M, Galli D, Glassgold AE. 2009. Cosmic-ray ionization of molecular clouds. Astron. Astrophys. 501, 619–631. ( 10.1051/0004-6361/200911794) [DOI] [Google Scholar]
- 77.Rimmer PB, Helling C. 2013. Ionization in atmospheres of brown dwarfs and extrasolar planets. IV. The effect of cosmic rays. Astrophys. J. 774, 108 ( 10.1088/0004-637X/774/2/108) [DOI] [Google Scholar]
- 78.Chadney JM, Galand M, Koskinen TT, Miller S, Sanz-Forcada J, Unruh YC, Yelle RV. 2016. EUV-driven ionospheres and electron transport on extrasolar giant planets orbiting active stars. Astron. Astrophys. 587, A87 ( 10.1051/0004-6361/201527442) [DOI] [Google Scholar]
- 79.Vorgul I, Kellett BJ, Cairns RA, Bingham R, Ronald K, Speirs DC, McConville SL, Gillespie KM, Phelps ADR. 2011. Cyclotron maser emission: stars, planets, and laboratory. Phys. Plasmas 18, 056501 ( 10.1063/1.3567420) [DOI] [Google Scholar]
- 80.Gérard JC, Singh V. 1982. A model of energy deposition of energetic electrons and EUV emission in the Jovian and Saturnian atmospheres and implications. JGR 87, 4525–4532. ( 10.1029/JA087iA06p04525) [DOI] [Google Scholar]
- 81.Gérard JC, Bonfond B, Gustin J, Grodent D, Clarke JT, Bisikalo D, Shematovich V. 2009. Altitude of Saturn's aurora and its implications for the characteristic energy of precipitated electrons. Geophys. Res. Lett. 36, L02202 ( 10.1029/2008gl036554) [DOI] [Google Scholar]
- 82.Rimmer PB, Herbst E, Morata O, Roueff E. 2012. Observing a column-dependent ζ in dense interstellar sources: the case of the Horsehead nebula. Astron. Astrophys. 537, A7 ( 10.1051/0004-6361/201117048) [DOI] [Google Scholar]
- 83.Rimmer PB, Herbst E, Morata O, Roueff E. 2012. Observing a column-dependent ζ in dense interstellar sources: the case of the Horsehead nebula. Astron. Astrophys. 537, A7 ( 10.1051/0004-6361/201117048) [DOI] [Google Scholar]
- 84.Koskinen TT, Yelle RV, Lavvas P, Lewis NK. 2010. Characterizing the thermosphere of HD209458b with UV transit observations. Astrophys. J. 723, 116–128. ( 10.1088/0004-637X/723/1/116) [DOI] [Google Scholar]
- 85.Mizus II, Alijah A, Zobov NF, Lodi L, Kyuberis AA, Yurchenko SN, Tennyson J, Polyansky OL. 2017. ExoMol molecular line lists – XX: a comprehensive line list for H3+. Mon. Not. R. Astron. Soc. 468, 1717–1725. ( 10.1093/mnras/stx502) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article does not contain any additional data.