Therapeutic trials in difficult to treat steroid sensitive nephrotic syndrome: challenges and future directions (original) (raw)
Abstract
Steroid sensitive nephrotic syndrome is a common condition in pediatric nephrology, and most children have excellent outcomes. Yet, 50% of children will require steroid-sparing agents due to frequently relapsing disease and may suffer consequences from steroid dependence or use of steroid-sparing agents. Several steroid-sparing therapeutic agents are available with few high quality randomized controlled trials to compare efficacy leading to reliance on observational data for clinical guidance. Reported trials focus on short-term outcomes such as time to first relapse, relapse rates up to 1–2 years of follow-up, and few have studied long-term remission. Trial designs often do not consider inter-individual variability, and differing response to treatments may occur due to heterogeneity in pathogenic mechanisms, and genetic and environmental influences. Strategies are proposed to improve the quantity and quality of trials in steroid sensitive nephrotic syndrome with integration of biomarkers, novel trial designs, and standardized outcomes, especially for long-term remission. Collaborative efforts among international trial networks will help move us toward a shared goal of finding a cure for children with nephrotic syndrome.
Keywords: Nephrotic syndrome, Idiopathic nephrotic syndrome, Steroid sensitive nephrotic syndrome, Frequently relapsing nephrotic syndrome, Steroid resistant nephrotic syndrome, Children, Randomized controlled trials, Clinical trials, Steroid-sparing agents
Introduction
Childhood idiopathic nephrotic syndrome (NS) is one of the most common conditions encountered by pediatric nephrologists globally. Incidence of NS has remained stable over the past 60 years at approximately 2.92 per 100,000 children per year [1]. Based on response to steroids, NS is traditionally categorized into broad groups, with steroid sensitive nephrotic syndrome (SSNS) representing 90% of children presenting with this condition [2].
Approximately 25% of these children will be effectively cured and have no further relapses after an initial course of steroids. The remainder go on to have relapsing disease and are characterized based on the pattern of relapses as infrequently relapsing, frequently relapsing (FR) or steroid dependent (SD) [2, 3]. There is significant inter-individual variability in clinical course and treatment response that cannot be predicted based on demographics, relapse pattern, or previous treatment [2]. There are now several lines of evidence supporting various mechanisms leading to NS which may explain the heterogeneity seen in this condition.
Several therapeutic agents are now routinely used in the treatment of FR- and SD-NS including cyclophosphamide, calcineurin inhibitors, mycophenolate, levamisole, and rituximab. A clear advantage of any single agent over the others is lacking, and there are few head to head randomized trials directly comparing these agents [4]. Hence, pediatric nephrologists often move through the therapeutic agents in a stepwise fashion that is more often based on historical, institutional, or physician proclivities driven by medication side effect profiles, drug access, and patient preference, rather than by scientific rationale or evidence [5–7].
Long-term prognosis for children with FR- or SD-NS is excellent, with most achieving long-term remission in adolescence, and kidney failure is rare [2]. With the average age of onset of NS at 3 years, however, many children could endure a decade or more of relapsing disease with repeated exposure to corticosteroids, steroid-sparing therapeutics, and their associated side effects. This ultimately leads to a significant health care burden and reduced quality of life and children would benefit from induction of long-term remission early in the disease course [2, 8].
Many barriers have impeded our progress towards achieving long-term, treatment-free, remission, or “cure” in SSNS. The ever elusive pathogenesis that limits opportunities for targeted drug development, the scarcity of well-designed, randomized control trials, our focus on short-term outcomes over long-term remission, and the lack of a universally accepted definition of long-term remission are some of the barriers [4].
In this review, we highlight proposed pathogenic mechanisms that may lead to NS and how this heterogeneity can impact treatment response and clinical trials. We review clinical trials in SSNS focused on the treatment of frequently relapsing and steroid dependent disease, discuss their limitations and how we might strengthen future trials. We conclude by discussing future directions and incorporation of recent scientific advances and novel trial methodology to move us forward toward finding a cure for children with NS.
Potential pathways leading to nephrotic syndrome
Genetic susceptibility
Pathogenesis of nephrotic syndrome is not fully understood and likely results from complex interactions between genetic, immune, and environmental factors. Detailed reviews of SSNS pathogenesis [9] and SSNS genetics were recently published [10]. Human leukocyte antigen (HLA) risk alleles and mutations in immune regulatory genes increase the risk of SSNS but appear insufficient to cause disease alone. A multiple hit model is proposed with genetic risk alleles interacting with unknown environmental factors resulting in immune dysregulation leading to podocyte dysfunction and proteinuria [11]. Genes linked to the podocyte and slit diaphragm are also implicated in SSNS and exert direct effects on podocyte integrity. While no monogenic forms of NS are reported to exclusively cause SSNS, mutations in PLCE1, NPHS1, and genes associated with the Rho GTPase regulatory pathway (MAG12, TNS2, DLC1, CDK20, ITSN1, and ITSN2), essential for maintaining podocyte cytoskeletal structure, manifest variable phenotypes including both steroid resistant (SR) and SSNS [12]. Recent exome and genome-wide association studies have identified risk alleles for SSNS, mainly located in the _HLA_-DQ and _HLA_-DR regions, with some risk loci located near genes associated with immune function (Table 1) [13–16]. Imputation of HLA alleles has identified a number of deleterious and protective classical HLA alleles [13–17]. In European cohorts, the _HLA_-DRB1*07:01-DQA1*02:_01_-DQB1*02:02 was most strongly associated with SSNS, whereas in Japanese cohorts, it was the _HLA_-DRB1*08:_02_-DQB1*03:02 locus, suggesting different risk alleles may be at play in different ancestral populations [13–15].
Table 1.
Genetic risk loci and study population associated with steroid sensitive nephrotic syndrome
| HLA risk loci | Classical HLA alleles | Non-HLA risk loci |
|---|---|---|
| HLA-DQA1 HLA-DQB1 HLA-DRB1 | Deleterious: HLA-DQA1*02:01 (European, South Asian) HLA-DRB1*07:01 (European, South Asian) HLA-DRB1*02 (European, South Asian) HLA-DRB1*08:02 (Japanese) HLA-DQB1*03:02 (Japanese) | Immune mediated: CALHM6/DSE (European)a TNFSF15 (Japanese) BTNL2 (African) |
| Other/unknown: PARM1 (European) CALHM6/DSE (European)a NPHS1 (Japanese, South Asian, African)b / KIRREL2 (Japanese)a | ||
| Protective: HLA-DQA1*01 (European) HLA-DQA1*01:03 (European)a _HLA-DRB1*13 (_European)a HLA-DRB1*13:02 (Japanese) HLA-DQB1*06:04 (Japanese) |
Immune dysregulation
The role of the immune system in nephrotic syndrome remains poorly understood. Most initial work centered around cell-mediated immunity and a role for T cells due to the spontaneous remission of nephrotic syndrome in children with measles, which is known to suppress cell-mediated immunity, and the remission of nephrotic syndrome in patients with Hodgkin’s lymphoma after treatment [18]. Favorable response to cyclophosphamide and calcineurin inhibitors, known to primarily target cell–mediated immunity, also supports a role for T cells [19, 20].
Studies have found a relative imbalance of various T cell subsets with a reduction in CD4 + and an increase in CD8 + circulating T cells during relapse, as well as upregulation of Th2-related cytokines, elevated Th17 cells, and Th17-related cytokines and increased expression of IL-17 (interleukin-17) in kidney biopsies [21–23]. A role of regulatory T cells (Tregs) is suggested with reduced numbers of Tregs in children with active nephrotic syndrome, a protective effect of direct Treg infusion or stimulation by IL-2 in animal models, and the association of nephrotic syndrome with immunodeficiency, polyendocrinopathy, and enteropathy (IPEX) syndrome, an X-linked disease due to a mutation of FOXP3 which inactivates Tregs [24–29].
B cells are also implicated with the success of rituximab in producing sustained remission, coupled with the finding of HLA genetic risk alleles, suggesting a role for the adaptive immune response. Reconstitution of switched memory B cells (cells that have undergone isotype switching from IgM to IgG antibody production) was the strongest predictor of relapse after rituximab in a study of 28 children, suggesting immune response to specific antigens may be responsible for relapses [30]. Relapses of nephrotic syndrome were also observed before reconstitution of circulating B cells [31]. Explanations for this phenomenon include failure to deplete B cells in all body compartments, such as the long-lived plasma cells residing in bone marrow, or a waning of alternative non-B cell–mediated modes of action such as rituximab’s hypothesized direct effect on the actin cytoskeleton of podocytes via cross-reactivity with SMPDL-3b, a sphingomyelin phosphodiesterase expressed by podocytes [32]. Ofatumumab, a fully humanized anti-CD20 monoclonal antibody, which binds to a different CD-20 epitope, is also effective in nephrotic syndrome, which opposes the SMPDL-3b cross-reactivity theory [33]. Curiously, the measles virus does not just cause immunosuppression by depleting memory T cells, but also via B cells and long-lived plasma cells. It leads to immunological amnesia, a reset of the immune system, where a significant proportion of preexisting antibodies are lost, with an overall reduction in antibody diversity [34].
Circulating glomerular permeability factors
A circulating glomerular permeability factor has long been suspected to play a role in the pathogenesis of NS and focal segmental glomerulosclerosis (FSGS), but a single causative circulating factor has not been identified. A number of putative pathogenic circulating factors have been proposed including heparanase, hemopexin, angiopoietin-like 4 (ANGPTL4), cardiotrophin-like cytokine-1 (CLC-1), radical oxygen species, and soluble urokinase plasminogen activator receptor (suPAR) [35, 36]. Most recently, anti-nephrin antibodies were reported in 29% of 41 children and 21 adults with minimal change disease during relapses, and the antibodies were absent or significantly reduced during remission [37]. This finding requires validation in larger populations and earlier in the course of NS as it may only represent a subset of SSNS.
Gene-environment interaction and triggers
Environmental factors associated with nephrotic syndrome include respiratory viruses, EBV, other infections, and allergens. The most common trigger of nephrotic syndrome relapses is upper respiratory tract infections, which precede 50–70% of relapses in SSNS [38, 39]. EBV is also a pathogen of interest, with evidence of recent EBV infection found in approximately 50% of patients presenting with nephrotic syndrome in a French cohort [40]. Increasing evidence for the role of B cells in nephrotic syndrome, and the association with polymorphisms in _HLA_-DQA1 and _HLA_-DQB1 associated with the ability to produce anti-EBNA-1 antibodies, lends some strength to this hypothesis [41].
Aeroallergens, food allergies, and insect bites are reported to trigger nephrotic syndrome relapses, and small case series have reported improvement on elemental and oligoantigenic diets suggesting a pathogenic role of allergic disease [42, 43]. The lack of seasonality to relapses or a proven benefit of mast cell stabilizers in nephrotic syndrome casts doubts [42, 44].
The range of mechanisms proposed to lead to NS suggests NS is a heterogeneous group of disorders leading to a common clinical phenotype of NS via dysfunction of the glomerular filtration barrier. Due to this heterogeneity, the perceived effect of treatments from clinical trials may not be generalizable. Selection of participants in trials may also bias outcomes and lead to negative trials even though a subgroup, for example those with antibodies, may benefit depending on the treatment mechanism. Often pediatric trials are not powered for subgroup analyses, and these effects can be overlooked.
Defining long-term remission and long-term outcomes
A focus on short-term outcomes in SSNS clinical trials and a lack of a robust, standard definition of long-term remission to be used in clinical trials, limits our ability to identify treatments leading to long-term remission in children with NS. Defining long-term remission in SSNS presents several challenges. Very few studies report the relapse-free duration that reliably predicts long term remission or “cure” in their cohorts. Among 63 patients with SSNS, the longer the period of remission, not unexpectedly, resulted in a lower risk of relapse. Even after 5 years of remission, however, the risk of relapse remained as high as 23%, and a relapse-free period that definitively protected from future relapse could not be identified [45]. While studies show a steady decrease in disease activity with increasing age, the historical notion of nephrotic syndrome resolving at puberty regardless of type of therapeutic intervention also remains unproven [46].
SSNS was once thought to almost universally remit in adolescence; however, long-term studies now dispute this, with 16 to 42% of patients going on to have relapses in adulthood [45–49]. Several factors proposed to be associated with increased risk of relapse in adulthood include, diagnosis before the age of 6, higher number of relapses per patient per year and cyclosporine use; however, no single factor consistently predicted relapse in adulthood across all long-term studies [45–48]. In our center, 631 children with NS (SSNS and SRNS) were followed for 2.1 to 6.6 years, and 80% achieved long-term remission and were discharged from the nephrology clinic before 18 years of age. Demographic or clinical factors, individually or in combination, could not predict the clinical course, need for second-line agents, or long-term remission [2]. Variation in factors associated with relapses in adulthood may be due to differences in disease severity leading to selection bias, definitions of “adulthood” and long-term remission, geographical location, and ethnicity.
Definitions of long-term remission are infrequently reported in studies. One study used a definition of remission as 3 years treatment-free [50]. At our own center, we typically discharge children from follow-up after 4 years of treatment-free remission; however, the duration likely varies by physician practice and clinical setting. A consistent definition of long-term remission for use in clinical and research settings will greatly enhance our ability to understand long-term efficacy of steroid-sparing agents.
Therapeutic trials in steroid sensitive nephrotic syndrome
Improving long-term remission rate after initial episode
Since the introduction of the 8-week steroid regimen, by the International Study of Kidney Disease in Children (ISKDC) in the 1970s, much effort was expended on determining the optimal steroid regimen at the initial presentation of NS. There was a move toward longer regimens of 12 weeks from the original ISKDC 8-week course after the 2007 Cochrane review found longer steroid courses resulted in higher rates of sustained remission at 12–24 months, without an increase in adverse effects [51]. Recent randomized control trials (RCTs) have called this into question, and some centers have returned to an 8-week prednisone course for the initial presentation of NS (see an in-depth review of the use of corticosteroids in NS [52]). The PREDNOS trial, with 237 children at first episode of SSNS, found no advantage of 16 weeks over 8 weeks of prednisolone. A multicenter trial of 255 children from Japan compared 2 months versus 6 months of prednisolone and also found no difference in time to first relapse or the incidence of frequently relapsing disease [53]. Although these findings provide evidence for the shorter 8-week steroid course, a study in 1988 by the Arbeitsgemeinschaft fur Padiatrische Nephrologie (APN) of 61 children presenting with INS found 4 weeks versus 8 weeks of steroids resulted in a significantly higher relapse rate and lower long-term remission suggesting further reduction of the initial course beyond 8 weeks is unlikely to be beneficial [54]. The recent updated 2020 Cochrane review has recommended no further resources should be invested in determining the optimal duration of the initial steroid course [55]. Nevertheless, questions remain whether certain subgroups, such as very young children before 4 years of age, who may be at higher risk of steroid dependency or frequent relapses, may benefit from longer courses of steroids at diagnosis [56]. A recent meta-analysis of 54 studies suggests, we have made only modest gains in achieving long-term remission after treatment of the initial presentation of nephrotic syndrome; the risk of relapses declined from 78.4% in 1945 to 66.2% in 2011 regardless of initial steroid treatment duration [1].
While steroid-only regimens are the backbone of treatment, the burden of steroid side effects can be significant. The Gesellschaft für Pädiatrische Nephrologie (GPN, formally APN) published a protocol for a non-inferiority trial comparing standard 12-week steroid course (prednisolone 60 mg/m2 daily for 6 weeks followed by 40 mg/m2 alternate days for 6 weeks) to a 12-week course of mycophenolate mofetil combined with a shorter course of prednisolone (60 mg/m2 daily until remission and then 2-week course of prednisolone 40 mg/m2 alternate days) for the initial presentation of NS and results are pending [57]. Three studies are also underway assessing the use of levamisole as an adjunct to corticosteroids for the treatment of the initial presentation of nephrotic syndrome (ClinicalTrials.gov #NCT02818738, EudraCT 2016–002,324-92, and 2017–001,025-41). A recent study in adults found tacrolimus monotherapy to be non-inferior to corticosteroids to induce remission in minimal change disease. While there are likely significant differences in the underlying pathophysiology of minimal change disease in adults compared to children, the success of a completely steroid free regimen remains noteworthy [58].
If alternate steroid-sparing treatment strategies at the initial presentation of nephrotic syndrome result in a larger proportion of children achieving long-term remission, this could be a significant step forward in management of SSNS. However, when 1 in 4 children achieve long-term remission with steroids alone, exposing this group to additional immunosuppression with potentially more serious adverse effects upfront, is difficult to justify. It is critical that we find ways to identify this group in advance to avoid additional unnecessary immunosuppression.
Relapses
The dose and duration of corticosteroids for NS relapses to optimize remission rates and minimize corticosteroid toxicities were recently reviewed [52]. While the optimal steroid regimen for relapse is yet to be identified, in the quest to achieve long-term remission for children with nephrotic syndrome, it is unlikely the dose or duration will substantially alter the course of NS [1]. Time and resources should be devoted to optimizing steroid-sparing regimens in order to increase the proportion with long-term remission.
Frequently relapsing and steroid-dependent SSNS
There are few head-to-head trials comparing steroid-sparing agents in FR- and SD-SSNS; thus, there is no clear benefit of any one drug over another (Table 2) as demonstrated in a systematic Cochrane review, which failed to identify difference in efficacy of calcineurin inhibitors, mycophenolate mofetil, levamisole, and alkylating agents. The authors highlighted the need for further studies [4].
Table 2.
Randomized control trials of steroid-sparing agents for frequently relapsing or steroid dependent nephrotic syndrome
| Drug | Study | Country | Population | N | Intervention | Control | Outcome | Follow up |
|---|---|---|---|---|---|---|---|---|
| Chlorambucil | Grupe 1976 [59] | USA | FR or SD-SSNS Age 3.5 to 15.5 years | 21 | Chlorambucil 0.1–0.2 mg/kg/day increased every 2 weeks until WCC < 1000 cm3 then stopped (av. dose 0.33 mg/kg, mean duration 9.7 weeks) + prednisolone as per control group continued until WCC > 5000/cm3 then weaned | Prednisolone 80–120 mg on alt. days for 2 months tapered over 4–6 weeks | At 1 year, 0% had relapsed in the chlorambucil group vs 100% in the control group | 1 year |
| APN 1982 [60] | Germany (multicenter) | FR or SD-SSNS, Age 2 to 16 years Biopsy proven MCD | 50 | Oral chlorambucil 0.15 mg/kg/day for 8 weeks Both groups: Prednisone for 4 weeks | Oral CPA 2 mg/kg/day for 8 weeks | Overall, 6% of SD-SSNS and 75% of FR-SSNS in sustained remission at 2.5 years (P < 0.001). There was no difference between treatment groups | 2.5 years | |
| Cyclophosphamide | Barratt 1970 [61] | UK | FR-SSNS Age < 14 years On maintenance prednisolone | 30 | CPA 3 mg/kg/day + prednisolone for 8 weeks then weaned as per control group | Prednisolone withdrawal over 8 weeks | 20% in CPA group vs 73% in control group relapsed at 16 weeks (P = 0.005) | 16 weeks |
| Chiu 1973 [62] | Canada | FR-SSNS Age 2 to 15 years Biopsy proven MCD | 23 | CPA 75 mg/m2/day for 16 wks. + prednisolone as per control group | Prednisolone 60 mg/m2/day (max 60 mg) until urine protein normal for 2 wks. then alt. days for 4 mos | 16.7% vs 91% relapsed in CPA vs control group during follow up (P < 0.01). | 2 years | |
| ISKDC 1974 [63] | Multicenter (23 sites, 12 countries) | FR-SSNS Age 12 weeks to 16 years | 63 (53 in analysis) | Oral CPA 5 mg/kg/day to induce WCC 3000–5000/mm3, then decreased to 1–3 mg/kg/day to maintain leukopenia (duration 6 weeks) + Prednisolone 10 mg/m2/day for first 10 days | Prednisone 40 mg/m2/day on 3 consecutive days out of 7 days for 180 days | 48% in CPA group and 88% in control group relapsed (no p value quoted) over a mean follow up period of 22 months. Average time to first relapse 120 ± 39 days in CPA group vs 81 ± 17 days in control group (P < 0.001) | 1.8 years | |
| Levamisole | BAPN 1991 [64] | UK | FR or SD-SSNS Age not specified (mean 8.3 ± 3.6 years) | 61 | Levamisole 2.5 mg/kg alt. days (max 150 mg) | Placebo | 45% in levamisole group vs 13% in control group were in remission at 112 days (P = 0.008). Ten of the 14 in remission on levamisole relapsed within 3 months of stopping the drug | 112 days |
| Dayal 1994 [65] | India | SSNS, initial episode or relapse | 61 | Levamisole 2–3 mg/kg/day twice a week for 1 year + prednisolone as per control group | Prednisolone 60 mg/m2/day for 4 weeks followed by 40 mg/m2/day alt. days for 4 weeks | At 2.5 yrs. 63% vs 43% were in remission in levamisole and control group (P = 0.11). Longer time to first relapse in levamisole group (12 vs 10.5 months, P = 0.10) | 2.5 years | |
| Donia 2005 [66] | Egypt | SD-SSNS in relapse Age 3 to 15 yrs Biopsy proven MCD | 40 | Levamisole 2.5 mg/kg on alt. days for 6 months Both groups: Prednisolone 2 mg/kg/day until remission, 1 mg/kg alt. days for 14 days then study medication started. Prednisolone weaned to 1 mg/kg alternate days for 14 days then weaned by 0.25 mg/kg ev. 14 days until stopped | IV CPA 500 mg/m2/month. for 6 months | At end of therapy 50% vs 45% were in remission in the levamisole and CPA group (P value not stated). Post-treatment 25% in each group, 15% vs 5% and 5% in each group were in remission at 6 months, 2 years and 4 years respectively (NS) | 4 years | |
| Al-Saran 2006 [67] | Saudi Arabia | FR or SD-SSNS Age < 14 years No previous non-steroid immuno-suppressants | 56 | Levamisole 2.5 mg/kg alt. days for 1 year | Low-dose prednisolone (< 0.5 mg/kg) on alt. days | Greater reduction in relapse rate in levamisole group (0.29 vs 0.11/patient/month, P < 0.01). 62.5% vs 0% were relapse-free at 2 years in levamisole and control group | 2 years | |
| Gruppen 2018 [68] | International multicenter (13 sites, 5 countries) | FR or SD-SSNS Age 2 to 18 years | 103 (99 modified ITT analysis) | Levamisole 2.5 mg/kg alt. days (max 150 mg). Started 3 to 21 days after remission and continued for 12 mos. or until relapse | Placebo alt. days | Similar relapse-free survival until 100 days. After 100 days, relapse-free survival greater in levamisole group (HR 0.22, 95% CI 0.11–0.43, P = 0.001) | 1 year | |
| Calcineurin inhibitors | Ponticelli 1993 [69] | Italy (multicenter) | FR or SD-SSNS Age 2 to 15 years No treatment with cytotoxic agents in past 2 years (included adults > 15 years – results for children only reported here) | 55 | CsA 6 mg/kg/day for 9 mos. (dose adjusted to trough level 200–600 ng/ml), tapered by 25% every mo. until discontinued at 12 months | CPA 2.5 mg/kg/day for 8 weeks. Prednisone 60 mg/m2/day until complete remission then 40 mg/m2 every other day for 4 weeks | 70% vs 68% in sustained remission at 9 months (NS) and 20% vs 68% at 2 years (P value not stated) in CsA and CPA group | 2 years |
| Mycophenolate | Gellermann 2013 [70] | Germany (15 sites) | FR ± SD-SSNS Biopsy proven MCD | 60 | MMF for 12 months followed by CsA for 12 months Cross-over RCT. Both groups: MMF 1000–1200 mg/m2/day adjusted to trough level 1.5–2.5 ug/ml. CsA 150 mg/m2/day adjusted to trough levels 80–100 ng/ml | MMF for 12 months followed by CsA for 12 months | Lower relapse rate and longer time to first relapse on CsA in 1st year (0.24 vs 1.10 relapses/pt/year, P = 0.03) but not in 2nd year 85% vs 64% relapse-free during treatment with CsA vs MMF (P = 0.06). Post hoc analysis: Pts with a MPA-AUC < 50 ug.h/ml had more relapses (mean 1.4 vs 0.27/year, P < 0.05) | 2 years |
| Dorresteijn 2008 [71] | Netherlands, Belgium (6 sites) | FR ± SD-SSNS Age < 18 years Biopsy proven MCD | 31 (24 in analysis) | MMF 1200 mg/m2/day (max 1g bid) for 12 months. Dose reduced by 25% if adverse effects or infection occurred. Further 25% reduction if adverse effects persisted | CsA 4–5 mg/kg/day for 12 months. Dose adjusted to achieve trough levels of 50–150 ug/L | Greater decline in eGFR (primary endpoint) in CsA vs MMF group (14 vs 6 ml/min/1.73 m2, P = 0.03). Greater percentage in remission in CsA group at 1 year (91% vs 58%, P = 0.06) | 1 year | |
| Sinha 2019 [72] | India | FR or SD-SSNS Age 6 to 18 years Requiring prednisolone < 1 mg/kg alt. days No previous levamisole, MMF, CNI, azathioprine, or RTX treatment and no CPA in past 6 months | 149 | MMF 750 to 1000 mg/m2/day for 1 year + prednisolone as per control group | Levamisole 2–2.5 mg/kg alt. days for 1 year Prednisolone 2 mg/kg/day until remission, then 1.5 mg/kg alt. days for 4 weeks, tapered by 0.25 mg/kg every 2 weeks (stopped at 12 to 14 weeks) | No difference in relapse rate (1.05 vs 1.34 relapses/person-year; P = 0.12) or sustained remission at 1 year (40.8% vs 34.2%, P = 0.20) in MMF vs levamisole groups In subset of 137 pts, followed for median of 43.0 months, no difference in long-term outcomes | 1 year | |
| Iijima 2022 [73] | Japan (27 sites) | FR or SD-SSNS 2 to 18 years | 78 | RTX 375 mg/m2 weekly × 4 weeks followed by MMF 1000–1200 mg/m2/day for 17 months | RTX 375 mg/m2 weekly × 4 weeks followed by placebo for 17 months | Non-significant increase in time to treatment failure (frequent relapses, SD or resistance, use of immunosuppressive agents, 784 vs 472.5 days, P = 0.07) and time to relapse (654 vs 320 days, P = 0.07) in MMF group | 1.5 years | |
| Rituximab | Ravani 2011 [74] | Italy | SSNS dependent on prednisolone and CNIs for at least 1 year Age 1 to 16 years | 54 | RTX 375 mg/m2 (1 dose only or if pt had signs of prednisolone or CNI toxicity 2 doses 2 weeks apart) At 30 days, prednisolone was tapered off by 0.3 mg/kg/week if proteinuria < 1 g/day. After 2 weeks, CNI decreased by 50% and withdrawn after a further 2 weeks | Prednisolone and CNI alone. Doses tapered as in the intervention group if proteinuria < 1 g/day | Less proteinuria (0.11 vs 0.36 g/day; P = 0.003) and lower risk of relapse (18.5% vs 48.1%, P=0.03) in RTX group at 3 months | 1 year |
| Iijima 2014 [75] Kamei 2017 (long-term data) [76] | Japan (9 sites) | Complicated FR or SD-SSNS (≥ 4 relapses in 12 months or SD in past 2 years) in relapse Diagnosed between age 1 and 18 years | 52 (48 in analysis) | RTX 375 mg/m2 (max 500 mg) weekly for 4 weeks Both groups: If on prednisolone at screening, 60 mg/m2/day (max 80 g) for 4 weeks, if not on prednisolone, 60 mg/m2/day until in remission for 3 days Prednisolone weaning for both groups: 60 mg/m2/day alt. days for 2 weeks, 30 mg/m2/day on alt. days for 2 weeks, 15 mg/m2/day on alt. days for 2 weeks Other immunosuppressants weaned and stopped | Placebo IV infusion at same frequency as RTX | Longer relapse-free survival (267 vs 101 days, P < 0.0001) in RTX group At 1 year, 30% in RTX vs 5% in control group were in remission Ninety-four percent of RTX group relapsed during long-term follow up (mean follow-up 59 months) | 1 year | |
| Ravani 2015 [77] | Italy (4 sites) non-inferiority trial | SDNS for 6–12 months In remission on high dose prednisolone (≥ 0.7 mg/kg/day) Age 1–16 years | 30 | RTX 375 mg/m2 single dose. Prednisone tapered as per control group | Prednisone tapered by 0.3 mg/kg/week starting at 30 days and withdrawn if proteinuria < 1 g/m3/day at the end of taper. Restarted if proteinuria ≥ 1 g/m2/day. Steroid-sparing agents (CNI or CPA) used at discretion of local investigators | At 3 months, proteinuria lower (ratio of means 0.58; 95% CI, 0.18 to 1.95). At 6 and 12 months, respectively, 50% and 25% of RTX group were in sustained remission without prednisolone/CNI. 14 of 15 patients in control group relapsed during prednisone taper | 3 months | |
| Ahn 2018 [78] | South Korea (8 sites) | SD-SSNS + CNI dependence > 2 yr Diagnosed < 18 and age < 24 yrs | 61 (51 in analysis) | RTX 375 mg/m2 (max 500 mg) single dose; second dose given if B cell depletion not achieved at 2 wks. (n = 9) + (steroids ± CNI) | Steroids ± CNI | At 6 mos. 74% vs 31% in remission in RTX and control group (P = 0.003). Longer median duration of remission in RTX (9.0 vs 2.9 mos., P = 0.004) | 6 months | |
| NEPHRUTIX 2018 [79] | France (multicenter) | FR-SSNS + highly steroid/CNI and/or MMF-dependent | 23 | RTX 375 mg/m2 × 2, 1 week apart. Other agents weaned as per control group | Placebo IV MMF stopped 1 week after first infusion, CNI tapered by 25% every 2 weeks after 2nd infusion, steroids decreased by 25% every 2 weeks until 5 to 7 mg alternate days | At 6 months, 90% vs 0% were in remission in RTX and control group (P value not stated) | 6 months | |
| RITURNS 2018 [80] | India | SD-SSNS Age 3 to 16 years No previous corticosteroid-sparing agent | 120 | RTX 375 mg/m2 (max 500 mg) 2 doses, 1 week apart + tapering doses of alt. day prednisolone | Tacrolimus 0.2 mg/kg/day (target trough level 5–7 ng/mL) + tapering doses of alt. day prednisolone | Ninety percent vs 63% in RTX and tacrolimus group were in sustained remission at 1 year. (P < 0.001). Median time to 1st relapse longer in RTX group (40 vs 20 weeks, P < 0.001) | 1 year | |
| Ravani 2021 [81] | Italy | SD-SSNS Age 2 to 24 yrs | 30 | RTX 375 mg/m2 single dose Both groups: 45 day run in MMF 1200 mg/1.73 m2/day + steroid withdrawal | MMF 350 mg/m2 bid | Trial stopped due to high rate of relapse in MMF group. Risk of relapse at 12 months higher in MMF group (80% vs 13%, P = 0.008) | 1 year | |
| Ofatumumab | Ravani 2021 [82] | Italy | SD-SSNS + CNI dependent Age 2 to 24 yrs | 140 | Ofatumumab 1.50 mg/1.73m2 single dose Both groups: steroid and CNI minimized during 1 mo run in period. CNI and steroids tapered and withdrawn within 60 days | RTX 375 mg/m2 single dose | No difference in relapse rate at 1 or 2 years (53% vs 51% at 1 year, OR 1.06; 95% CI 0.55 to 2.06) | 2 years |
| Mizoribine | Yoshioka 2000 [83] | Japan (57 sites) | FR-SSNS Age 2 to 19 years | 197 | Mizoribine 4 mg/kg/day for 48 week | Placebo Prednisolone 1–2 mg/kg for 28 days, then tapered and stopped by 12 weeks | Non-significant lower relapse rate in mizoribine group (0.0055 vs. 0.0067 relapses/day, P = 0.12). Subgroup analysis of < 10 y.o., relapse rate significantly lower (ratio 0.66, 95% CI 0.44 to 0.94; P = 0.017) | 18 months |
Low-dose alternate day corticosteroids are another common approach to FR-SSNS. Only two controlled trials have been performed comparing this strategy to steroid-sparing agents. Both compared cyclophosphamide to the low-dose steroid strategy, with cyclophosphamide clearly the superior drug [62, 63].
Cyclophosphamide has been used in SSNS for over 50 years, but just five randomized controlled trials have compared cyclophosphamide to placebo, steroids alone, or other steroid-sparing agents. The first UK trial in 1970 was conducted in 30 children with FR-SSNS on maintenance prednisolone. Cyclophosphamide of 3 mg/kg/day for 8 weeks with prednisolone withdrawal compared to prednisolone withdrawal alone was associated with a significant reduction in relapse risk at 16 weeks (20% vs. 73%, P = 0.005) [61]. Two subsequent trials in Canada and Europe compared cyclophosphamide to placebo demonstrating significant improvements in relapse rates up to 2 years [62, 63]. Only two RCTs compared cyclophosphamide to other steroid-sparing agents. In 1982, the APN compared cyclophosphamide to chlorambucil in 50 children and found no difference in sustained remission up to 2.5 years, and in 1993, an Italian group demonstrated no differences in relapses up to 9 months on cyclophosphamide 2.5 mg/kg/day for 8 weeks to cyclosporine 6 mg/kg/day [69, 84]. Recently, a non-randomized pilot study in Saudi Arabia compared rituximab to cyclophosphamide (rituximab 375 mg/m2 two doses vs. cyclophosphamide 3 mg/kg/day for 8 weeks) as the first steroid-sparing agent in 46 children with FR- and SD-SSNS, and showed a non-significant increase in 1-year relapse-free survival in the rituximab group (84.2% vs. 58.6%, P = 0.1).
Cyclophosphamide has the advantage of achieving long-term remission in approximately 30% of children after a single course and 58% will have ≤ 2 relapses, based on observational studies, over an average follow-up of 4 years [85]. There is a fear of potential serious long-term adverse events such as cancer and infertility after cyclophosphamide with limited data [86]. Nonetheless, physician preference due to these concerns limits its use [5]. There is, however, a distinct advantage over other second agents where relapses tend to recur after discontinuation of the drug.
Calcineurin inhibitors, despite their widespread use, have just three randomized control trials to support their use. Two studies used cyclosporine and were compared to cyclophosphamide and mycophenolate mofetil. Mycophenolate mofetil (1200 mg/m2/day) was compared to cyclosporine A (trough level target 50–150ug/L) over 12 months, in a multi-center study in Belgium and the Netherlands in children with FR- and SD-SSNS. Although a greater percentage in the cyclosporine group were in remission at 12 months (91% vs. 58%), the study was underpowered with only 31 patients and the finding not statistically significant [71]. The primary outcome in the study was eGFR decline, which showed a small but significantly greater decline in eGFR at 1 year among those on cyclosporine vs. mycophenolate (14 vs. 6 ml/min/1.73 m2). When we consider the additional mechanism of cyclosporine and risk of a small and reversible drop in GFR, the relevance of this finding to the long-term outcomes is unclear. One RCT included tacrolimus as the control arm and found it to be inferior to rituximab in maintaining sustained remission at 12 months [80].
Levamisole is also effective in reducing the risk of relapse at 12 months on meta-analysis (HR 0.22, 95% CI 0.11–0.43), although the time to first relapse is similar in the first 100 days on the therapy [68]. In six trials of levamisole, the most-studied steroid-sparing agent in trials, three compared levamisole to placebo or prednisolone withdrawal, one to low-dose prednisolone and two to other steroid-sparing agents (IV cyclophosphamide and mycophenolate mofetil). While effective compared to placebo and low-dose prednisolone, no difference in efficacy was found compared to cyclophosphamide or mycophenolate [64–68, 72]. Most trials assessing levamisole were performed in South Asia and the Middle East where the drug is readily available.
Mycophenolate mofetil is the most common steroid-sparing drug used in Europe by physician preference as it is appealing for its lack of nephrotoxicity [6]. Three RCTs found mycophenolate mofetil less effective in reducing relapse rates and achieving sustained remission compared to cyclosporine; however, the recent Cochrane meta-analysis found no difference in relapse at 12 months (RR 1.9, 95% CI 0.66–5.46) [4]. A criticism of these RCTs is the use of standard dosing regimens (750–1200 mg/m2/day) or trough levels to adjust mycophenolate mofetil doses which correlate poorly with mycophenolic acid area under the concentration–time curve (MPA-AUC). A post hoc analysis of an RCT of 60 children, which initially reported poorer outcomes in patients on mycophenolate mofetil vs. cyclosporine A, found patients with high mycophenolic acid exposure (MPA-AUC > 50 ug.h/ml) had fewer relapses compared to those with lower mycophenolic acid exposure (MPA-AUC < 50ug.h/ml) and similar relapse-free survival in those with higher mycophenolic acid exposure compared to those on cyclosporine A [70]. An MPA-AUC > 45 ug/h/ml was also associated with lower risk of relapse in two retrospective observational studies [87, 88]. This raises the question of whether the mycophenolate mofetil efficacy can be improved with therapeutic drug monitoring. Limited sampling AUC strategies using either Bayesian estimation or multiple linear regression are validated in patients with nephrotic syndrome, and prospective trials are needed to assess utility of integration into clinical practice [89]. Low-dose mycophenolate strategies have also been tried; however, a recent RCT comparing low-dose mycophenolate (350 mg/m2 bid) to rituximab was stopped after randomization of just 30 patients due to unacceptably high rate of relapse in the mycophenolate group [81].
Rituximab has emerged as a very effective steroid-sparing agent in the short term. In an RCT of 120 children with SDNS, rituximab significantly improved 12-month relapse-free survival when compared to tacrolimus (90 vs. 63.3%; P < 0.001) [80]. However, the risk of recurrence after discontinuation and subsequent B cell repopulation is high, and in a multinational cohort study of 421 children, 81% relapsed after rituximab therapy (median relapse-free survival of 12.5 months) [30, 90]. Relapse-free survival may be increased with the adjunct of maintenance immunosuppression after rituximab, specifically mycophenolate [90]. A recent multicenter RCT compared rituximab followed by mycophenolate for 6 months to rituximab and placebo and found no significant difference in treatment failure (defined as development of frequent relapses, steroid dependent or resistance, or use of immunosuppressive agents or rituximab) or time to first relapse. Post hoc analysis did show a reduction in relapse rate during the mycophenolate course (HR 0.26; 95% CI 0.08 to 0.48) [73]. Rituximab was commonly used as a third- or fourth-line agent due to concerns of potential serious adverse effects including progressive multifocal leukoencephalopathy and persistent hypogammaglobulinemia, but with increasing experience in its use, studies using rituximab earlier in the course of the disease are underway [91]. Research for other monoclonal antibodies is limited — a single RCT has been published assessing ofatumumab and found it was not superior to rituximab [82]. Whether other selected antibodies can change the natural history of nephrotic syndrome and achieve long-term remission remains unknown.
Limitations of current trials
Over the past 60 years, only 24 controlled trials investigated the seven most used medications in FR- and SD-SSNS (Table 2). Most trials were small with a median of 55 children. Small study population size only allows for detection of large treatment effects, and precludes the ability to identify subgroups of this heterogeneous disease that have either a beneficial effect, no consequence or harm from specific treatments [92]. Most trials are also limited to a single geographical area, which limits the generalizability of the findings due to the known geographical and genetic differences in SSNS, and the outcomes reported are not consistent, and hence, it is not possible to directly compare outcomes [85]. Trials also focus on short-term outcomes with the longest trial follow-up of a maximum of 2.5 years, although some trials report subsequent observational periods. Our focus on short-term outcomes is insufficient, and inclusion of long-term outcomes is needed to change the life course of disease. In the context of high cost and large time commitment to conduct RCTs, observational studies have a role in informing practice but remain vulnerable to selection bias, even after the use of rigorous statistical techniques to reduce potential bias (i.e. propensity methods).
Future directions
RCTs remain the gold-standard in assessing efficacy of therapeutics, and we must commit to improving the availability of quality randomized evidence in NS. We have highlighted the challenges to performing these trials in SSNS, including (1) unknown pathogenic mechanisms limiting targeted drug discovery; (2) high degree of heterogeneity in the SSNS population; (3) inadequate sample sizes; and (4) a lack of standardized outcomes. Nephrology as a whole lags behind other specialties in quantity and quality of clinical trials, but strategies are available to advance NS trials [93].
Addressing heterogeneity and identifying biomarkers
The ability to identify subgroups of SSNS that are similar in either underlying pathological mechanisms or prognosis will provide opportunities to enrich clinical trials through selection of participants that are most likely to benefit from a specific drug, increasing the likelihood of detecting a drug effect [94]. There are early examples of using genomics to predict response in SSNS. In 1997, a German retrospective study of 54 children with FR-NS + / − SD-NS found _HLA-DR7_-positive children were less likely to remain in remission after cyclophosphamide or chlorambucil than _HLA-DR7_-negative children (36% vs. 81% in remission at 3 years). HLA-DR7 status was better at predicting a favorable response to alkylating agents than the rate of previous relapses [95]. This allele was identified as a risk factor for SSNS in European and South Asian cohorts, suggesting a role in the pathogenesis and response to treatment [13, 14, 17]. A study of 113 children with NS found the GR-9 polymorphism of the glucocorticoid receptor gene, related to impaired glucocorticoid sensitivity, was associated with a higher risk of steroid dependence. These studies illustrate the need to consider an individual’s responsiveness to treatment, and its influence on outcomes, in clinical trials. To date, neither HLA typing nor pharmacogenetics have been incorporated into prospective therapeutic trials in SSNS.
Genomics, transcriptomics, metabolomics, and proteomics in combination with machine learning show promise in defining homogeneous subgroups within glomerular disorders. Recently, researchers were able to categorize adults and children with FSGS or minimal change disease (MCD) into 3 clusters associated with complete remission and eGFR decline using a digital pathology scoring system and unsupervised machine learning techniques [96]. The glomerular transcriptome data found a cluster associated with poorest clinical outcome showed downregulation of podocyte specific gene expression and an increase in monocyte/macrophage specific gene expression, laying the groundwork to identify potential biomarkers and therapeutic targets [96].
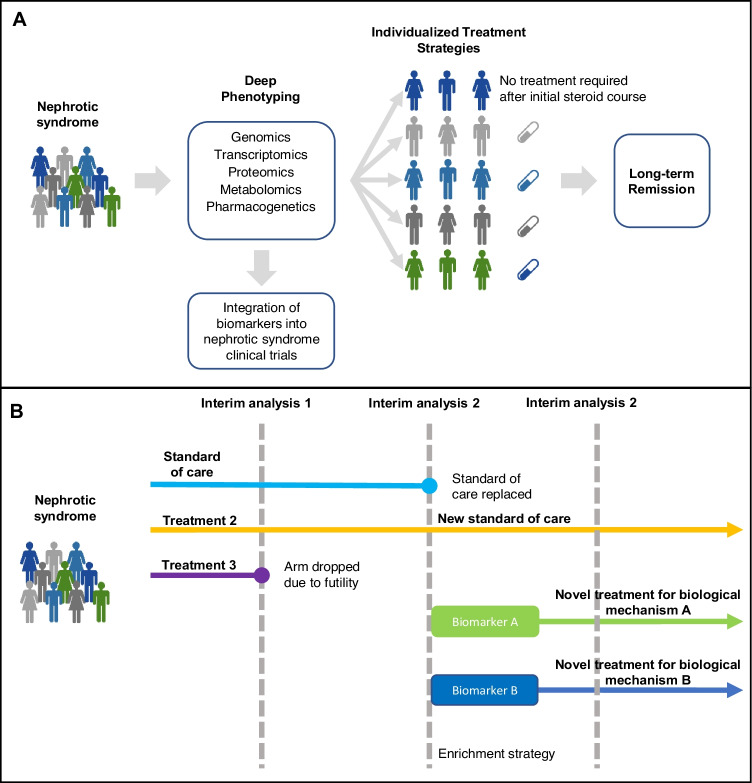
A number of nephrotic syndrome cohort studies are in process with many incorporating multi-omic approaches. The Nephrotic Syndrome Study Network (NEPTUNE) study in North America, a study of children and adults with MCD, FSGS, and membranous nephropathy, has already published several studies using these techniques [97]. Other cohort studies are ongoing, such as the Canadian childhood nephrotic syndrome (CHILDNEPH) study and the insight into nephrotic syndrome (INSIGHT) study in Canada and the European Rare Kidney Disease Registry (ERKReg) in Europe [98, 99]. With a greater understanding of the molecular mechanisms, and identification of biomarkers that predict clinical course and treatment response in SSNS, we can aim for precision medicine, where treatment is tailored to the individual child’s genomic, molecular and pharmacogenetic profile (Fig. 1A).
Fig. 1.
A Potential precision medicine model for steroid sensitive nephrotic syndrome. Current phenotyping of steroid sensitive nephrotic syndrome is limited to clinical features. In the future, deep phenotyping may be available based on genetic variants and biomolecular profiles. Biomarkers can be incorporated into clinical trials to identify children who will benefit most from existing and new therapies, ultimately leading to a precision medicine approach where treatment is personalized to achieve the optimal outcome for each individual child. B Graphical example of an adaptive platform trial with integration of biomarkers. Pre-specified changes can be made during planned interim analyses of trial data. Treatment arms may be dropped due to futility, a superior treatment can replace the standard of care, and new treatment arms can be introduced as novel agents become available and biomarker enrichment strategies can be used
Novel trial designs
Traditional RCTs are expensive and time consuming, which has led to the development of novel, more efficient trial designs. Master protocol trials test multiple interventions in one or multiple diseases, in a single protocol, and are a more efficient trial design [100]. They encompass umbrella trials that assess multiple interventions in a single disease, basket trials that assess a single intervention within multiple diseases, or disease subtypes, and platform trials that assess multiple interventions in a disease (often within biomarker- and clinically defined subgroups) in a perpetual manner [100, 101]. Adaptive platform trials allow iterative adjustments to the master protocol when pre-specified criteria are met and can include changes in sample size, enrichment strategies, and addition of treatment arms when new drugs are available, or cessation if treatments are deemed futile (Fig. 1B).
The COVID-19 pandemic highlighted the value added from novel trial designs. The Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial is an adaptive platform trial assessing multiple interventions for patients hospitalized with COVID-19 in the UK. The trial began in early 2020 and has identified several effective (dexamethasone, tocilizumab and casirivimab/imdevimab) and six ineffective treatments [102–104]. This is a remarkable achievement in 2 years and illustrates the potential of platform trials to rapidly produce high-quality data and adapt to new medications as they become available. During the pandemic, broad data–sharing policies and collaborative research team models involving industry, scientists, patients, and researchers demonstrated the value in collaboration to reduce inefficiencies, find innovative solutions, and enhance knowledge translation of research findings.
Standardized trial outcomes
The use of a standard set of outcomes in SSNS research allows comparison across trials and reduces research waste. The Standardised Outcomes in Nephrology (SONG) initiative is working toward establishing a set of validated core outcomes, through input from both clinicians and patients. Core outcome measures are under development for children with chronic kidney disease and for patients with glomerular disease, both of which will inform standard outcomes measures for NS research [105, 106]. We also advocate for the inclusion of a standard definition of long-term remission to ensure we remain focused on this important outcome.
Trial networks and patient engagement
Strong trial networks facilitate patient recruitment, build clinical trial capacity, and minimize research waste by reducing duplication. The National Institute of Cancer–sponsored Children’s Oncology Group (COG) is an example of an extremely successful trial network in pediatrics. It includes centers from North America, Australia, New Zealand, and Europe, and over 90% of children with cancer in the USA are treated at a COG center. COG has an enviable trial participation rate, peaking at 50–70% of all US pediatric cancer patients enrolled in clinical trials in 1990s [107]. Their research efforts have been largely responsible for improving survival rates in childhood cancer, from an almost incurable disease, to over 80% 5-year survival [108].
In response to the deficiency of high-quality clinical trials in nephrology, the International Society of Nephrology formed the Advancing Clinical Trials initiative (ISN-ACT 2013) to support clinical trial capacity–building worldwide [109, 110]. Nephrology clinical trial networks now exist across the world and include the UK Renal Trials Network, Canadian Nephrology Trials Network, Australasian Kidney Trials Network, and the Global Kidney Patient Trials Network started by the George Institute. Leveraging these existing resources can help accelerate research, but greater gains are likely if strong international collaborations can be developed in NS research, to ensure trial results are generalizable given the known geographical and genetic differences in this disease [85]. Also, NS is a rare disease and requires patient advocacy to engage patients and increase trial participation and selection of relevant patient outcomes such as long-term remission [111, 112].
Conclusion
Children with difficult to treat SSNS, despite good long-term outcomes, can endure a decade or more of medical intervention. Steroid-sparing agents available to treat this condition lack well-designed and sufficiently powered controlled trials to guide treatment choice. There is not a single steroid-sparing agent shown to be superior and choice is often dependent on region, clinical practice, and physician preference. Heterogeneity in pathogenic mechanisms, genetics, and environment influences in SSNS can impact trial design and treatment response. Integration of biomarkers, use of novel trial design, and standardized outcomes including a standardized definition of long-term remission have the potential to improve clinical trials and outcomes in NS. Further collaborative international trial networks are needed to work toward a shared goal of finding a cure for children with nephrotic syndrome.
Author contribution
The idea was conceived by Rulan Parekh. The literature search was performed and the first draft of the manuscript was written by Ashlene McKay. All authors critically revised the manuscript.
Funding
This research was undertaken, in part, thanks to funding from the Canada Research Chairs program.
Declarations
Competing interest
Rulan Parekh is the principal investigator for the INSIGHT study. Ashlene McKay and Damien Noone declare no conflicts of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Veltkamp F, Rensma LR, Bouts AHM. Incidence and relapse of idiopathic nephrotic syndrome: meta-analysis. Pediatrics. 2021;148:e2020029249. doi: 10.1542/peds.2020-029249. [DOI] [PubMed] [Google Scholar]
- 2.Carter SA, Mistry S, Fitzpatrick J, Banh T, Hebert D, Langlois V, Pearl RJ, Chanchlani R, Licht CPB, Radhakrishnan S, Brooke J, Reddon M, Levin L, Aitken-Menezes K, Noone D, Parekh RS. Prediction of short- and long-term outcomes in childhood nephrotic syndrome. Kidney Int Rep. 2020;5:426–434. doi: 10.1016/j.ekir.2019.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kidney disease: improving global outcomes (KDIGO) Glomerulonephritis Work Group KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Inter Suppl. 2012;2:139–274. [Google Scholar]
- 4.Larkins NG, Liu ID, Willis NS, Craig JC, Hodson EM. Non-corticosteroid immunosuppressive medications for steroid-sensitive nephrotic syndrome in children. Cochrane Database Syst Rev. 2020;4:CD002290. doi: 10.1002/14651858.CD002290.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hladunewich MA, Beanlands H, Herreshoff E, Troost JP, Maione M, Trachtman H, Poulton C, Nachman P, Modes MM, Hailperin M, Pitter R, Gipson DS. Provider perspectives on treatment decision-making in nephrotic syndrome. Nephrol Dial Transplant. 2017;32:i106–i114. doi: 10.1093/ndt/gfw309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deschênes G, Vivarelli M, Peruzzi L. Variability of diagnostic criteria and treatment of idiopathic nephrotic syndrome across European countries. Eur J Pediatr. 2017;176:647–654. doi: 10.1007/s00431-017-2891-2. [DOI] [PubMed] [Google Scholar]
- 7.MacHardy N, Miles PV, Massengill SF, Smoyer WE, Mahan JD, Greenbaum L, Massie S, Yao L, Nagaraj S, Lin JJ, Wigfall D, Trachtman H, Hu Y, Gipson DS. Management patterns of childhood-onset nephrotic syndrome. Pediatr Nephrol. 2009;24:2193–2201. doi: 10.1007/s00467-009-1282-y. [DOI] [PubMed] [Google Scholar]
- 8.Li N, Hao J, Fu T, Du Y. Evaluating the quality of life of 231 children with primary nephrotic syndrome and assessing parental awareness of the disease. Front Pediatr. 2021;9:745444. doi: 10.3389/fped.2021.745444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horinouchi T, Nozu K, Iijima K. An updated view of the pathogenesis of steroid-sensitive nephrotic syndrome. Pediatr Nephrol. 2022 doi: 10.1007/s00467-021-05401-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dufek-Kamperis S, Kleta R, Bockenhauer D, Gale D, Downie ML. Novel insights in the genetics of steroid-sensitive nephrotic syndrome in childhood. Pediatr Nephrol. 2021;36:2165–2175. doi: 10.1007/s00467-020-04780-4. [DOI] [PubMed] [Google Scholar]
- 11.Karp AM, Gbadegesin RA. Genetics of childhood steroid-sensitive nephrotic syndrome. Pediatr Nephrol. 2017;32:1481–1488. doi: 10.1007/s00467-016-3456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lane BM, Cason R, Esezobor CI, Gbadegesin RA. Genetics of childhood steroid sensitive nephrotic syndrome: an update. Front Pediatr. 2019;7:8. doi: 10.3389/fped.2019.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dufek S, Cheshire C, Levine AP, Trompeter RS, Issler N, Stubbs M, Mozere M, Gupta S, Klootwijk E, Patel V, Hothi D, Waters A, Webb H, Tullus K, Jenkins L, Godinho L, Levtchenko E, Wetzels J, Knoers N, Teeninga N, Nauta J, Shalaby M, Eldesoky S, Kari JA, Thalgahagoda S, Ranawaka R, Abeyagunawardena A, Adeyemo A, Kristiansen M, Gbadegesin R, Webb NJ, Gale DP, Stanescu HC, Kleta R, Bockenhauer D. Genetic identification of two novel loci associated with steroid-sensitive nephrotic syndrome. J Am Soc Nephrol. 2019;30:1375–1384. doi: 10.1681/ASN.2018101054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debiec H, Dossier C, Letouze E, Gillies CE, Vivarelli M, Putler RK, Ars E, Jacqz-Aigrain E, Elie V, Colucci M, Debette S, Amouyel P, Elalaoui SC, Sefiani A, Dubois V, Simon T, Kretzler M, Ballarin J, Emma F, Sampson MG, Deschenes G, Ronco P. Transethnic, genome-wide analysis reveals immune-related risk alleles and phenotypic correlates in pediatric steroid-sensitive nephrotic syndrome. J Am Soc Nephrol. 2018;29:2000–2013. doi: 10.1681/ASN.2017111185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia X, Horinouchi T, Hitomi Y, Shono A, Khor SS, Omae Y, Kojima K, Kawai Y, Nagasaki M, Kaku Y, Okamoto T, Ohwada Y, Ohta K, Okuda Y, Fujimaru R, Hatae K, Kumagai N, Sawanobori E, Nakazato H, Ohtsuka Y, Nakanishi K, Shima Y, Tanaka R, Ashida A, Kamei K, Ishikura K, Nozu K, Tokunaga K, Iijima K. Strong association of the HLA-DR/DQ locus with childhood steroid-sensitive nephrotic syndrome in the Japanese population. J Am Soc Nephrol. 2018;29:2189–2199. doi: 10.1681/ASN.2017080859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia X, Yamamura T, Gbadegesin R, McNulty MT, Song K, Nagano C, Hitomi Y, Lee D, Aiba Y, Khor SS, Ueno K, Kawai Y, Nagasaki M, Noiri E, Horinouchi T, Kaito H, Hamada R, Okamoto T, Kamei K, Kaku Y, Fujimaru R, Tanaka R, Shima Y, Research consortium on genetics of childhood idiopathic nephrotic syndrome in Japan Common risk variants in NPHS1 and TNFSF15 are associated with childhood steroid-sensitive nephrotic syndrome. Kidney Int. 2020;98:1308–1322. doi: 10.1016/j.kint.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adeyemo A, Esezobor C, Solarin A, Abeyagunawardena A, Kari JA, El Desoky S, Greenbaum LA, Kamel M, Kallash M, Silva C, Young A, Hunley TE, de Jesus-Gonzalez N, Srivastava T, Gbadegesin R. HLA-DQA1 and APOL1 as risk loci for childhood-onset steroid-sensitive and steroid-resistant nephrotic syndrome. Am J Kidney Dis. 2018;71:399–406. doi: 10.1053/j.ajkd.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shalhoub R. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet. 1974;2:556–560. doi: 10.1016/s0140-6736(74)91880-7. [DOI] [PubMed] [Google Scholar]
- 19.Lin C, Hsu H. Histopathological and immunological studies in spontaneous remission of nephrotic syndrome after intercurrent measles infection. Nephron. 1986;42:10–115. doi: 10.1159/000183647. [DOI] [PubMed] [Google Scholar]
- 20.Audard V, Larousserie F, Grimbert P, Abtahi M, Sotto JJ, Delmer A, Boue F, Nochy D, Brousse N, Delarue R, Remy P, Ronco P, Sahali D, Lang P, Hermine O. Minimal change nephrotic syndrome and classical Hodgkin’s lymphoma: report of 21 cases and review of the literature. Kidney Int. 2006;69:2251–2260. doi: 10.1038/sj.ki.5000341. [DOI] [PubMed] [Google Scholar]
- 21.Yap H, Cheung W, Murugasu B, Sim S, Seah C, Jordan S. Th1 and Th2 cytokine mRNA profiles in childhood nephrotic syndrome: evidence for increased IL-13 mRNA expression in relapse. J Am Soc Nephrol. 1999;10:529–537. doi: 10.1681/ASN.V103529. [DOI] [PubMed] [Google Scholar]
- 22.Lama G, Luongo I, Tirino G, Borriello A, Carangio C, Salsano ME. T-lymphocyte populations and cytokines in childhood nephrotic syndrome. Am J Kidney Dis. 2002;39:958–965. doi: 10.1053/ajkd.2002.32769. [DOI] [PubMed] [Google Scholar]
- 23.Shao XS, Yang XQ, Zhao XD, Li Q, Xie YY, Wang XG, Wang M, Zhang W. The prevalence of Th17 cells and FOXP3 regulate T cells (Treg) in children with primary nephrotic syndrome. Pediatr Nephrol. 2009;24:1683–1690. doi: 10.1007/s00467-009-1194-x. [DOI] [PubMed] [Google Scholar]
- 24.Bertelli R, Bodria M, Nobile M, Alloisio S, Barbieri R, Montobbio G, Patrone P, Ghiggeri GM. Regulation of innate immunity by the nucleotide pathway in children with idiopathic nephrotic syndrome. Clin Exp Immunol. 2011;166:55–63. doi: 10.1111/j.1365-2249.2011.04441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertelli R, Di Donato A, Cioni M, Grassi F, Ikehata M, Bonanni A, Rastaldi MP, Ghiggeri GM. LPS nephropathy in mice is ameliorated by IL-2 independently of regulatory T cells activity. PLoS ONE. 2014;9:e111285. doi: 10.1371/journal.pone.0111285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Berre L, Bruneau S, Naulet J, Renaudin K, Buzelin F, Usal C, Smit H, Condamine T, Soulillou J-P, Dantal J. Induction of T regulatory cells attenuates idiopathic nephrotic syndrome. J Am Soc Nephrol. 2009;20:57–67. doi: 10.1681/ASN.2007111244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertelli R, Trivelli A, Magnasco A, Cioni M, Bodria M, Carrea A, Montobbio G, Barbano G, Ghiggeri GM. Failure of regulation results in an amplified oxidation burst by neutrophils in children with primary nephrotic syndrome. Clin Exp Immunol. 2010;161:151–158. doi: 10.1111/j.1365-2249.2010.04160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashimura Y, Nozu K, Kanegane H, Miyawaki T, Hayakawa A, Yoshikawa N, Nakanishi K, Takemoto M, Iijima K, Matsuo M. Minimal change nephrotic syndrome associated with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome. Pediatr Nephrol. 2009;24:1181–1186. doi: 10.1007/s00467-009-1119-8. [DOI] [PubMed] [Google Scholar]
- 29.Bertelli R, Bonanni A, Di Donato A, Cioni M, Ravani P, Ghiggeri GM. Regulatory T cells and minimal change nephropathy: in the midst of a complex network. Clin Exp Immunol. 2016;183:166–174. doi: 10.1111/cei.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colucci M, Carsetti R, Cascioli S, Casiraghi F, Perna A, Rava L, Ruggiero B, Emma F, Vivarelli M. B cell reconstitution after rituximab treatment in idiopathic nephrotic syndrome. J Am Soc Nephrol. 2016;27:1811–1822. doi: 10.1681/asn.2015050523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato M, Kamei K, Ogura M, Ishikura K, Ito S. Relapse of nephrotic syndrome during post-rituximab peripheral blood B-lymphocyte depletion. Clin Exp Nephrol. 2018;22:110–116. doi: 10.1007/s10157-017-1415-8. [DOI] [PubMed] [Google Scholar]
- 32.Fornoni A, Sageshima J, Wei C, Merscher-Gomez S, Aguillon-Prada R, Jauregui AN, Li J, Mattiazzi A, Ciancio G, Chen L, Zilleruelo G, Abitbol C, Chandar J, Seeherunvong W, Ricordi C, Ikehata M, Rastaldi MP, Reiser J, Burke GW., 3rd Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med. 2011;3:85ra46. doi: 10.1126/scitranslmed.3002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basu B. Ofatumumab for rituximab-resistant nephrotic syndrome. New Engl J Med. 2014;370:1268–1270. doi: 10.1056/NEJMc1401725. [DOI] [PubMed] [Google Scholar]
- 34.Mina MJ, Kula T, Leng Y, Li M, de Vries RD, Knip M, Siljander H, Rewers M, Choy DF, Wilson MS, Larman HB, Nelson AN, Griffin DE, de Swart RL, Elledge SJ. Measles virus infection diminishes preexisting antibodies that offer protection from other pathogens. Science. 2019;366:599–606. doi: 10.1126/science.aay6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davin JC. The glomerular permeability factors in idiopathic nephrotic syndrome. Pediatr Nephrol. 2016;31:207–215. doi: 10.1007/s00467-015-3082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maas RJ, Deegens JK, Wetzels JF. Serum suPAR in patients with FSGS: trash or treasure? Pediatr Nephrol. 2013;28:1041–1048. doi: 10.1007/s00467-013-2452-5. [DOI] [PubMed] [Google Scholar]
- 37.Watts AJB, Keller KH, Lerner G, Rosales I, Collins AB, Sekulic M, Waikar SS, Chandraker A, Riella LV, Alexander MP, Troost JP, Chen J, Fermin D, Yee JL, Sampson MG, Beck LH, Jr, Henderson JM, Greka A, Rennke HG, Weins A. Discovery of autoantibodies targeting nephrin in minimal change disease supports a novel autoimmune etiology. J Am Soc Nephrol. 2022;33:238–252. doi: 10.1681/asn.2021060794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi S, Wada N, Murakami H, Funaki S, Inagaki T, Harada K, Nagata M. Triggers of relapse in steroid-dependent and frequently relapsing nephrotic syndrome. Pediatr Nephrol. 2007;22:232–236. doi: 10.1007/s00467-006-0316-y. [DOI] [PubMed] [Google Scholar]
- 39.MacDonald N, Wolfish N, McLaine P, Phipps P, Rossier E. Role of respiratory viruses in exacerbations of primary nephrotic syndrome. J Pediatr. 1986;108:378–382. doi: 10.1016/s0022-3476(86)80876-9. [DOI] [PubMed] [Google Scholar]
- 40.Dossier C, Sellier-Leclerc AL, Rousseau A, Michel Y, Gautheret-Dejean A, Englender M, Madhi F, Charbit M, Ulinski T, Simon T, Jacqz-Aigrain E, Deschênes G. Prevalence of herpes viruses at onset of idiopathic nephrotic syndrome. Pediatr Nephrol. 2014;29:2325–2331. doi: 10.1007/s00467-014-2860-1. [DOI] [PubMed] [Google Scholar]
- 41.Dossier C, Jamin A, Deschenes G. Idiopathic nephrotic syndrome: the EBV hypothesis. Pediatr Res. 2017;81:233–239. doi: 10.1038/pr.2016.200. [DOI] [PubMed] [Google Scholar]
- 42.Abdel-Hafez M, Shimada M, Lee P, Johnson R, Garin E. Idiopathic nephrotic syndrome and atopy: is there a common link? Am J Kidney Dis. 2009;54:945–953. doi: 10.1053/j.ajkd.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandberg D, Bernstein C, McIntosh R, Carr R, Strauss J. Severe steroid-responsive nephrosis associated with hypersensitivity. Lancet. 1977;1:388–391. doi: 10.1016/s0140-6736(77)92603-4. [DOI] [PubMed] [Google Scholar]
- 44.Riar SS, Banh THM, Borges K, Subbarao P, Patel V, Vasilevska-Ristovska J, Chanchlani R, Hussain-Shamsy N, Noone D, Hebert D, Licht CPB, Langlois V, Pearl RJ, Parekh RS. Prevalence of asthma and allergies and risk of relapse in childhood nephrotic syndrome: insight into nephrotic syndrome cohort. J Pediatr. 2019;208(251–257):e251. doi: 10.1016/j.jpeds.2018.12.048. [DOI] [PubMed] [Google Scholar]
- 45.Lewis MA, Baildom EM, Davis N, Houtston IB, Pstlethwaite RJ. Nephrotic syndrome: from toddlers to twenties. Lancet. 1989;333:255–259. doi: 10.1016/s0140-6736(89)91266-x. [DOI] [PubMed] [Google Scholar]
- 46.Fakhouri F, Bocquet N, Taupin P, Presne C, Gagnadoux MF, Landais P, Lesavre P, Chauveau D, Knebelmann B, Broyer M, Grunfeld JP, Niaudet P. Steroid-sensitive nephrotic syndrome: from childhood to adulthood. Am J Kidney Dis. 2003;41:550–557. doi: 10.1053/ajkd.2003.50116. [DOI] [PubMed] [Google Scholar]
- 47.Trompeter RS, Lloyd BW, Hicks J, White RH, Cameron JS. Long-term outcome for children with minimal-change nephrotic syndrome. Lancet. 1985;325:368–370. doi: 10.1016/s0140-6736(85)91387-x. [DOI] [PubMed] [Google Scholar]
- 48.Ruth EM, Kemper MJ, Leumann EP, Laube GF, Neuhaus TJ. Children with steroid-sensitive nephrotic syndrome come of age: long-term outcome. J Pediatr. 2005;147:202–207. doi: 10.1016/j.jpeds.2005.03.050. [DOI] [PubMed] [Google Scholar]
- 49.Skrzypczyk P, Panczyk-Tomaszewska M, Roszkowska-Blaim M, Wawer Z, Bienias B, Zajgzkowska M, Kilis-Pstrusinska K, Jakubowska A, Szczepaniak M, Pawlak-Bratkowska M, Tkaczyk M. Long-term outcomes in idiopathic nephrotic syndrome: from childhood to adulthood. Clin Nephrol. 2014;81:166–173. doi: 10.5414/cn108044. [DOI] [PubMed] [Google Scholar]
- 50.Kabuki N, Okugawa T, Hayakawa H, Tomizawa S, Kasahara T, Uchiyama M. Influence of age at onset on the outcome of steroid-sensitive nephrotic syndrome. Pediatr Nephrol. 1998;12:467–470. doi: 10.1007/s004670050489. [DOI] [PubMed] [Google Scholar]
- 51.Hodson EM, Willis NS, Craig JC. Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database Syst Rev. 2007;4:CD001533. doi: 10.1002/14651858.CD001533.pub4. [DOI] [PubMed] [Google Scholar]
- 52.Christian MT, Maxted AP. Optimizing the corticosteroid dose in steroid-sensitive nephrotic syndrome. Pediatr Nephrol. 2022;37:37–47. doi: 10.1007/s00467-021-04985-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshikawa N, Nakanishi K, Sako M, Oba MS, Mori R, Ota E, Ishikura K, Hataya H, Honda M, Ito S, Shima Y, Kaito H, Nozu K, Nakamura H, Igarashi T, Ohashi Y, Iijima K. A multicenter randomized trial indicates initial prednisolone treatment for childhood nephrotic syndrome for two months is not inferior to six-month treatment. Kidney Int. 2015;87:225–232. doi: 10.1038/ki.2014.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arbeitsgemeinschaft fur Padiatrische Nephrologie (APN) Short versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome. Lancet. 1988;331:380–383. [PubMed] [Google Scholar]
- 55.Hahn D, Samuel SM, Willis NS, Craig JC, Hodson EM. Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database Syst Rev. 2020;8:CD001533. doi: 10.1002/14651858.CD001533.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hiraoka M, Tsukahara H, Matsubara K, Tsurusawa M, Takeda N, Haruki S, Hayashi S, Ohta K, Momoi T, Ohshima Y, Suganuma N, Mayumi M. A randomized study of two long-course prednisolone regimens for nephrotic syndrome in children. Am J Kidney Dis. 2003;41:1155–1162. doi: 10.1016/s0272-6386(03)00346-9. [DOI] [PubMed] [Google Scholar]
- 57.Ehren R, Benz MR, Doetsch J, Fichtner A, Gellermann J, Haffner D, Hocker B, Hoyer PF, Kastner B, Kemper MJ, Konrad M, Luntz S, Querfeld U, Sander A, Toenshoff B, Weber LT, Gesellschaft fur PadiatrischeNephrologie (GPN) Initial treatment of steroid-sensitive idiopathic nephrotic syndrome in children with mycophenolate mofetil versus prednisone: protocol for a randomised, controlled, multicentre trial (INTENT study) BMJ Open. 2018;8:e024882. doi: 10.1136/bmjopen-2018-024882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Medjeral-Thomas NR, Lawrence C, Condon M, Sood B, Warwicker P, Brown H, Pattison J, Bhandari S, Barratt J, Turner N, Cook HT, Levy JB, Lightstone L, Pusey C, Galliford J, Cairns TD, Griffith M. Randomized, controlled trial of tacrolimus and prednisolone monotherapy for adults with de novo minimal change disease: a multicenter, randomized, controlled trial. Clin J Am Soc Nephrol. 2020;15:209–218. doi: 10.2215/cjn.06180519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grupe W, Makker S, Ingelfinger J. Chlorambucil treatment of frequently relapsing nephrotic syndrome. N Engl J Med. 1976;295:746–749. doi: 10.1056/NEJM197609302951402. [DOI] [PubMed] [Google Scholar]
- 60.Arbeitsgemeinschaft fur Padiatrische Nephrologie Effect of cytotoxic drugs in frequently relapsing nephrotic syndrome with and without steroid dependence. N Engl J Med. 1982;306:451–454. doi: 10.1056/NEJM198202253060803. [DOI] [PubMed] [Google Scholar]
- 61.Barratt T, Soothill J. Controlled trial of cyclophosphamide in steroid-sensitive relapsing nephrotic syndrome of childhood. Lancet. 1970;296:479–482. doi: 10.1016/s0140-6736(70)90108-x. [DOI] [PubMed] [Google Scholar]
- 62.Chiu J, McLaine P, Drummond K. A controlled prospective study of cyclophosphamide in relapsing, corticosteroid-responsive, minimal-lesion nephrotic syndrome in childhood. J Pediatr. 1973;82:607–613. doi: 10.1016/s0022-3476(73)80585-2. [DOI] [PubMed] [Google Scholar]
- 63.(1974) Prospective, controlled trial of cyclophosphamide therapy in children with nephrotic syndrome. Report of the International study of Kidney Disease in Children. Lancet 304:423–427 [PubMed]
- 64.(1991) Levamisole for corticosteroid-dependent nephrotic syndrome in childhood. British Association for Paediatric Nephrology. Lancet 337:1555–1557 [PubMed]
- 65.Dayal U, Dayal A, Shastry J, Raghupathy P. Use of levamisole in maintaining remission in steroid-sensitive nephrotic syndrome in children. Nephron. 1994;66:408–412. doi: 10.1159/000187855. [DOI] [PubMed] [Google Scholar]
- 66.Donia AF, Ammar HM, Ael-B E-A, Fel-H M, Sobh MA. Long-term results of two unconventional agents in steroid-dependent nephrotic children. Pediatr Nephrol. 2005;20:1420–1425. doi: 10.1007/s00467-005-1943-4. [DOI] [PubMed] [Google Scholar]
- 67.Al-Saran K, Mirza K, Al-Ghanam G, Abdelkarim M. Experience with levamisole in frequently relapsing, steroid-dependent nephrotic syndrome. Pediatr Nephrol. 2006;21:201–205. doi: 10.1007/s00467-005-2080-9. [DOI] [PubMed] [Google Scholar]
- 68.Gruppen MP, Bouts AH, Jansen-van der Weide MC, Merkus MP, Zurowska A, Maternik M, Massella L, Emma F, Niaudet P, Cornelissen EAM, Schurmans T, Raes A, van de Walle J, van Dyck M, Gulati A, Bagga A, Davin JC. A randomized clinical trial indicates that levamisole increases the time to relapse in children with steroid-sensitive idiopathic nephrotic syndrome. Kidney Int. 2018;93:510–518. doi: 10.1016/j.kint.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 69.Ponticelli C, Edefonti A, Ghio L, Rizzoni G, Rinaldi S, Gusmano R, Lama G, Zacchello G, Confalonieri R, Altieri P, et al. Cyclosporin versus cyclophosphamide for patients with steroid-dependent and frequently relapsing idiopathic nephrotic syndrome: a multicentre randomized controlled trial. Nephrol Dial Transplant. 1993;8:1326–1332. [PubMed] [Google Scholar]
- 70.Gellermann J, Weber L, Pape L, Tönshoff B, Hoyer P, Querfeld U. Mycophenolate mofetil versus cyclosporin A in children with frequently relapsing nephrotic syndrome. J Am Soc Nephrol. 2013;24:1689–1697. doi: 10.1681/asn.2012121200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dorresteijn EM, Kist-van Holthe JE, Levtchenko EN, Nauta J, Hop WC, van der Heijden AJ. Mycophenolate mofetil versus cyclosporine for remission maintenance in nephrotic syndrome. Pediatr Nephrol. 2008;23:2013–2020. doi: 10.1007/s00467-008-0899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sinha A, Puraswani M, Kalaivani M, Goyal P, Hari P, Bagga A. Efficacy and safety of mycophenolate mofetil versus levamisole in frequently relapsing nephrotic syndrome: an open-label randomized controlled trial. Kidney Int. 2019;95:210–218. doi: 10.1016/j.kint.2018.08.039. [DOI] [PubMed] [Google Scholar]
- 73.Iijima K, Sako M, Oba M, Tanaka S, Hamada R, Sakai T, Ohwada Y, Ninchoji T, Yamamura T, Machida H, Shima Y, Tanaka R, Kaito H, Araki Y, Morohashi T, Kumagai N, Gotoh Y, Ikezumi Y, Kubota T, Kamei K, Fujita N, Ohtsuka Y, Okamoto T, Yamada T, Tanaka E, Shimizu M, Horinochi T, Konishi A, Omori T, Nakanishi K, Ishikura K, Ito S, Nakamura H, Nozu K, Japanese Study Group of Kidney Disease in Children Mycophenolate mofetil after rituximab for childhood-onset complicated frequently-relapsing or steroid-dependent nephrotic syndrome. J Am Soc Nephrol. 2022;33:401–419. doi: 10.1681/ASN.2021050643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ravani P, Magnasco A, Edefonti A, Murer L, Rossi R, Ghio L, Benetti E, Scozzola F, Pasini A, Dallera N, Sica F, Belingheri M, Scolari F, Ghiggeri GM. Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: a randomized controlled trial. Clin J Am Soc Nephrol. 2011;6:1308–1315. doi: 10.2215/cjn.09421010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iijima K, Sako M, Nozu K, Mori R, Tuchida N, Kamei K, Miura K, Aya K, Nakanishi K, Ohtomo Y, Takahashi S, Tanaka R, Kaito H, Nakamura H, Ishikura K, Ito S, Ohashi Y. Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2014;384:1273–1281. doi: 10.1016/s0140-6736(14)60541-9. [DOI] [PubMed] [Google Scholar]
- 76.Kamei K, Ishikura K, Sako M, Aya K, Tanaka R, Nozu K, Kaito H, Nakanishi K, Ohtomo Y, Miura K, Takahashi S, Morimoto T, Kubota W, Ito S, Nakamura H, Iijima K. Long-term outcome of childhood-onset complicated nephrotic syndrome after a multicenter, double-blind, randomized, placebo-controlled trial of rituximab. Pediatr Nephrol. 2017;32:2071–2078. doi: 10.1007/s00467-017-3718-0. [DOI] [PubMed] [Google Scholar]
- 77.Ravani P, Rossi R, Bonanni A, Quinn RR, Sica F, Bodria M, Pasini A, Montini G, Edefonti A, Belingheri M, De Giovanni D, Barbano G, Degl’Innocenti L, Scolari F, Murer L, Reiser J, Fornoni A, Ghiggeri GM. Rituximab in children with steroid-dependent nephrotic syndrome: a multicenter, open-label, noninferiority, randomized controlled trial. J Am Soc Nephrol. 2015;26:2259–2266. doi: 10.1681/asn.2014080799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ahn YH, Kim SH, Han KH, Choi HJ, Cho H, Lee JW, Shin JI, Cho MH, Lee JH, Park YS, Ha IS, Cheong HI, Kim SY, Lee SJ, Kang HG. Efficacy and safety of rituximab in childhood-onset, difficult-to-treat nephrotic syndrome: a multicenter open-label trial in Korea. Medicine. 2018;97:e13157. doi: 10.1097/md.0000000000013157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boumediene A, Vachin P, Sendeyo K, Oniszczuk J, Zhang SY, Henique C, Pawlak A, Audard V, Ollero M, Guigonis V, Sahali D. NEPHRUTIX: a randomized, double-blind, placebo vs rituximab-controlled trial assessing T-cell subset changes in minimal change nephrotic syndrome. J Autoimmun. 2018;88:91–102. doi: 10.1016/j.jaut.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 80.Basu B, Sander A, Roy B, Preussler S, Barua S, Mahapatra TKS, Schaefer F. Efficacy of rituximab vs tacrolimus in pediatric corticosteroid-dependent nephrotic syndrome: a randomized clinical trial. JAMA Pediatr. 2018;172:757–764. doi: 10.1001/jamapediatrics.2018.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ravani P, Lugani F, Drovandi S, Caridi G, Angeletti A, Ghiggeri GM. Rituximab vs low-dose mycophenolate mofetil in recurrence of steroid-dependent nephrotic syndrome in children and young adults: a randomized clinical trial. JAMA Pediatr. 2021;175:631–632. doi: 10.1001/jamapediatrics.2020.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ravani P, Colucci M, Bruschi M, Vivarelli M, Cioni M, DiDonato A, Cravedi P, Lugani F, Antonini F, Prunotto M, Emma F, Angeletti A, Ghiggeri GM. Human or chimeric monoclonal anti-CD20 antibodies for children with nephrotic syndrome: a superiority randomized trial. J Am Soc Nephrol. 2021;32:2652–2663. doi: 10.1681/ASN.2021040561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yoshioka K, Ohashi Y, Sakai T, Ito H, Yoshikawa N, Nakamura H, Tanizawa T, Wada H, Maki S. A multicenter trial of mizoribine compared with placebo in children with frequently relapsing nephrotic syndrome. Kidney Int. 2000;58:317–324. doi: 10.1046/j.1523-1755.2000.00168.x. [DOI] [PubMed] [Google Scholar]
- 84.Arbeitsgemeinschaft für Pädiatrische Nephrologie Effect of cytotoxic drugs in frequently relapsing nephrotic syndrome with and without steroid dependence. N Engl J Med. 1982;306:451–454. doi: 10.1056/NEJM198202253060803. [DOI] [PubMed] [Google Scholar]
- 85.Banh TH, Hussain-Shamsy N, Patel V, Vasilevska-Ristovska J, Borges K, Sibbald C, Lipszyc D, Brooke J, Geary D, Langlois V, Reddon M, Pearl R, Levin L, Piekut M, Licht CP, Radhakrishnan S, Aitken-Menezes K, Harvey E, Hebert D, Piscione TD, Parekh RS. Ethnic differences in incidence and outcomes of childhood nephrotic syndrome. Clin J Am Soc Nephrol. 2016;11:1760–1768. doi: 10.2215/CJN.00380116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Samuel SM, Flynn R, Zappitelli M, Dart A, Parekh R, Pinsk M, Mammen C, Wade A, Scott SD. Factors influencing practice variation in the management of nephrotic syndrome: a qualitative study of pediatric nephrology care providers. CMAJ Open. 2017;5:E424–E430. doi: 10.9778/cmajo.20160078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tellier S, Dallocchio A, Guigonis V, Saint-Marcoux F, Llanas B, Ichay L, Bandin F, Godron A, Morin D, Brochard K, Gandia P, Bouchet S, Marquet P, Decramer S, Harambat J. Mycophenolic acid pharmacokinetics and relapse in children with steroid-dependent idiopathic nephrotic syndrome. Clin J Am Soc Nephrol. 2016;11:1777–1782. doi: 10.2215/CJN.00320116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hackl Á, Cseprekál O, Gessner M, Liebau MC, Habbig S, Ehren R, Müller C, Taylan C, Dötsch J, Weber LT. Mycophenolate mofetil therapy in children with idiopathic nephrotic syndrome. Ther Drug Monit. 2016;38:274–279. doi: 10.1097/FTD.0000000000000258. [DOI] [PubMed] [Google Scholar]
- 89.Sobiak J, Resztak M, Ostalska-Nowicka D, Zachwieja J, Gąsiorowska K, Piechanowska W, Chrzanowska M. Monitoring of mycophenolate mofetil metabolites in children with nephrotic syndrome and the proposed novel target values of pharmacokinetic parameters. Eur J Pharm Sci. 2015;77:189–196. doi: 10.1016/j.ejps.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 90.Chan EY, Webb H, Yu E, Ghiggeri GM, Kemper MJ, Ma AL, Yamamura T, Sinha A, Bagga A, Hogan J, Dossier C, Vivarelli M, Liu ID, Kamei K, Ishikura K, Saini P, Tullus K. Both the rituximab dose and maintenance immunosuppression in steroid-dependent/frequently-relapsing nephrotic syndrome have important effects on outcomes. Kidney Int. 2020;97:393–401. doi: 10.1016/j.kint.2019.09.033. [DOI] [PubMed] [Google Scholar]
- 91.Nagano C, Sako M, Kamei K, Ishikura K, Nakamura H, Nakanishi K, Omori T, Nozu K, Iijima K. Study protocol: multicenter double-blind, randomized, placebo-controlled trial of rituximab for the treatment of childhood-onset early-stage uncomplicated frequently relapsing or steroid-dependent nephrotic syndrome (JSKDC10 trial) BMC Nephrol. 2019;20:293. doi: 10.1186/s12882-019-1470-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kent DM, Paulus JK, van Klaveren D, D’Agostino R, Goodman S, Hayward R, Ioannidis JPA, Patrick-Lake B, Morton S, Pencina M, Raman G, Ross JS, Selker HP, Varadhan R, Vickers A, Wong JB, Steyerberg EW. The Predictive Approaches to Treatment effect Heterogeneity (PATH) statement. Ann Intern Med. 2020;172:35–45. doi: 10.7326/m18-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Inrig JK, Califf RM, Tasneem A, Vegunta RK, Molina C, Stanifer JW, Chiswell K, Patel UD. The landscape of clinical trials in nephrology: a systematic review of Clinicaltrials.gov. Am J Kidney Dis. 2014;63:771–780. doi: 10.1053/j.ajkd.2013.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.U.S. Food and Drug Administration (2019) Enrichment strategies for clinical trials to support determination of effectiveness of human drugs and biological products. Guidance for Industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enrichment-strategies-clinical-trials-support-approval-human-drugs-and-biological-products
- 95.Konrad M, Mytilineos J, Ruder H, Opelz G, Schärer K. HLA-DR7 predicts the response to alkylating agents in steroid-sensitive nephrotic syndrome. Pediatr Nephrol. 1997;11:16–19. doi: 10.1007/s004670050224. [DOI] [PubMed] [Google Scholar]
- 96.Hodgin JB, Mariani LH, Zee J, Liu Q, Smith AR, Eddy S, Hartman J, Hamidi H, Gaut JP, Palmer MB, Nast CC, Chang A, Hewitt S, Gillespie BW, Kretzler M, Holzman LB, Barisoni L. Quantification of glomerular structural lesions: associations with clinical outcomes and transcriptomic profiles in nephrotic syndrome. Am J Kidney Dis. 2021 doi: 10.1053/j.ajkd.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gadegbeku CA, Gipson DS, Holzman LB, Ojo AO, Song PXK, Barisoni L, Sampson MG, Kopp JB, Lemley KV, Nelson PJ, Lienczewski CC, Adler SG, Appel GB, Cattran DC, Choi MJ, Contreras G, Dell KM, Fervenza FC, Gibson KL, Greenbaum LA, Hernandez JD, Hewitt SM, Hingorani SR, Hladunewich M, Hogan MC, Hogan SL, Kaskel FJ, Lieske JC, Meyers KEC, Nachman PH, Nast CC, Neu AM, Reich HN, Sedor JR, Sethna CB, Trachtman H, Tuttle KR, Zhdanova O, Zilleruelo GE, Kretzler M. Design of the Nephrotic Syndrome Study Network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int. 2013;83:749–756. doi: 10.1038/ki.2012.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Samuel SM, Dart A, Filler G, Bitzan M, Pinsk M, Mammen C, Nettel-Aguirre A, Perinpanayagam MA, Takano T, Chanchlani R, Zappitelli M. The Canadian childhood nephrotic syndrome (CHILDNEPH) study: report on mid-study feasibility, recruitment and main measures. BMC Nephrol. 2019;20:159. doi: 10.1186/s12882-019-1320-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hussain N, Zello JA, Vasilevska-Ristovska J, Banh TM, Patel VP, Patel P, Battiston CD, Hebert D, Licht CP, Piscione TD, Parekh RS. The rationale and design of insight into nephrotic syndrome: investigating genes, health and therapeutics (INSIGHT): a prospective cohort study of childhood nephrotic syndrome. BMC Nephrol. 2013;14:25. doi: 10.1186/1471-2369-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Adaptive Platform Trials Coalition Adaptive platform trials: definition, design, conduct and reporting considerations. Nat Rev Drug Discov. 2019;18:797–807. doi: 10.1038/s41573-019-0034-3. [DOI] [PubMed] [Google Scholar]
- 101.Woodcock J, LaVange LM. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med. 2017;377:62–70. doi: 10.1056/NEJMra1510062. [DOI] [PubMed] [Google Scholar]
- 102.Lim PC, Wong KL, Rajah R, Chong MF, Chow TS, Subramaniam S, Lee CY. Comparing the efficacy of tocilizumab with corticosteroid therapy in treating COVID-19 patients: a systematic review and meta-analysis. Daru. 2022 doi: 10.1007/s40199-021-00430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.RECOVERY Collaborative Group Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/s0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.RECOVERY Collaborative Group, Horby PW, Mafham M, Peto L, Campbell M, Pessoa-Amorim G, Spata E, Staplin N, Emberson JR, Prudon B, Hine P, Brown T, Green CA, Sarkar R, Desai P, Yates B, Bewick T, Tiberi S, Felton T, Baillie JK, Buch MH, Chappell LC, Day JN, Faust SN, Jaki T, Jeffery K, Juszczak E, Lim WS, Montgomery A, Mumford A, Rowan K, Thwaites G, Weinreich DM, Haynes R, Landray MJ (2021) Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. https://www.medrxiv.org/content/10.1101/2021.06.15.21258542v1
- 105.Carter SA, Lightstone L, Cattran D, Bagga A, Barbour SJ, Barratt J, Boletis J, Caster D, Coppo R, Fervenza FC, Floege J, Hladunewich M, Hogan JJ, Kitching AR, Lafayette R, Malvar A, Radhakrishnan J, Rovin BH, Zhang H, Gutman T, Howell M, Logeman C, Shen JI, Teixeira-Pinto A, Alexander SI, Cho Y, Craig JC, Harris D, Johnson DW, Kerr PG, Ryan J, Viecelli AK, Wang AY, Wilkie M, Scholes-Robertson N, Tong A. Standardized Outcomes in Nephrology-Glomerular Disease (SONG-GD): establishing a core outcome set for trials in patients with glomerular disease. Kidney Int. 2019;95:1280–1283. doi: 10.1016/j.kint.2019.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tong A, Samuel S, Zappitelli M, Dart A, Furth S, Eddy A, Groothoff J, Webb NJ, Yap HK, Bockenhauer D, Sinha A, Alexander SI, Goldstein SL, Gipson DS, Hanson CS, Evangelidis N, Crowe S, Harris T, Hemmelgarn BR, Manns B, Gill J, Tugwell P, Van Biesen W, Wheeler DC, Winkelmayer WC, Craig JC. Standardised Outcomes in Nephrology-Children and Adolescents (SONG-Kids): a protocol for establishing a core outcome set for children with chronic kidney disease. Trials. 2016;17:401. doi: 10.1186/s13063-016-1528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Faulk KE, Anderson-Mellies A, Cockburn M, Green AL. Assessment of enrollment characteristics for Children’s Oncology Group (COG) upfront therapeutic clinical trials 2004–2015. PLoS ONE. 2020;15:e0230824–e0230824. doi: 10.1371/journal.pone.0230824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.American Cancer Society (2022 ) Key statistics for childhood cancers. https://www.cancer.org/cancer/cancer-in-children/key-statistics.html. Accessed 28 January 2022
- 109.Levin A, Tonelli M, Bonventre J, Coresh J, Donner JA, Fogo AB, Fox CS, Gansevoort RT, Heerspink HJL, Jardine M, Kasiske B, Köttgen A, Kretzler M, Levey AS, Luyckx VA, Mehta R, Moe O, Obrador G, Pannu N, Parikh CR, Perkovic V, Pollock C, Stenvinkel P, Tuttle KR, Wheeler DC, Eckardt KU. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet. 2017;390:1888–1917. doi: 10.1016/s0140-6736(17)30788-2. [DOI] [PubMed] [Google Scholar]
- 110.Evans RDR, Smyth B, Levin A, Jha V, Wheeler DC, Jardine M, Perkovic V, Damster S, Malik C, de Zeeuw D, Hiemstra T. The International Society of Nephrology Advancing Clinical Trials (ISN-ACT) network: current activities and future goals. Kidney Int. 2021;99:551–554. doi: 10.1016/j.kint.2020.10.048. [DOI] [PubMed] [Google Scholar]
- 111.Crocker JC, Ricci-Cabello I, Parker A, Hirst JA, Chant A, Petit-Zeman S, Evans D, Rees S. Impact of patient and public involvement on enrolment and retention in clinical trials: systematic review and meta-analysis. BMJ. 2018;363:k4738. doi: 10.1136/bmj.k4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brett J, Staniszewska S, Mockford C, Herron-Marx S, Hughes J, Tysall C, Suleman R. Mapping the impact of patient and public involvement on health and social care research: a systematic review. Health Expect. 2014;17:637–650. doi: 10.1111/j.1369-7625.2012.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]