Cytopathic Killing of Peripheral Blood CD4+ T Lymphocytes by Human Immunodeficiency Virus Type 1 Appears Necrotic rather than Apoptotic and Does Not Require env (original) (raw)
Abstract
An important unresolved issue of AIDS pathogenesis is the mechanism of human immunodeficiency virus (HIV)-induced CD4+ T-lymphocyte destruction. We show here that HIV type 1 (HIV-1) exerts a profound cytopathic effect upon peripheral blood CD4+ T lymphocytes that resembles necrosis rather than apoptosis. Necrotic cytopathology was found with both laboratory-adapted strains and primary isolates of HIV-1. We carefully investigated the role of env, which has been previously implicated in HIV cytopathicity. HIV-1 stocks with equivalent infectivity were prepared from constructs with either an intact or mutated env coding region and pseudotyped with the glycoprotein of vesicular stomatitis virus (VSV-G) so that the HIV envelope was not rate-limiting for infection. Infected Jurkat T cells died whether or not env was intact; however, the expression of env accelerated death significantly. The accelerated death was blocked by protease inhibitors, indicating that it was due to reinfection by newly produced virus in env+ cultures. Accordingly, we found no disparity in kinetics in CD4lo Jurkat cells. In highly infected peripheral blood T cells, profound necrosis occurred equivalently with both env+ and env− stocks of HIV-1. We also found that HIV-1 cytopathicity was undiminished by the absence of nef. However, viral stocks made by complementation or packaging of HIV-1 genomes with the natural protein-coding sequences replaced by the green fluorescent protein were highly infectious but not cytopathic. Thus, env can accelerate cell death chiefly as an entry function, but one or more viral functions other than env or nef is essential for necrosis of CD4+ T cells induced by HIV-1.
The experimental definition of viral cytopathicity leading to the demise of the host cell was established in poliovirus (22). The observation that poliovirus caused a severe biochemical derangement of the host cell machinery so that the cell would die provided an important insight into viral pathogenesis. Infected cell death and dysfunction are frequently associated with organ damage and are believed to play an important part of virus-induced disease pathology (84). Cytopathic effects have been observed for many viruses including the human immunodeficiency virus (HIV) (68). Extensive cell death after infection impeded the initial attempts to propagate HIV in tissue culture (68).
The cytopathic effect of HIV on T cells has been the subject of substantial investigation, and several hypotheses have been previously advanced (for reviews, see references 24, 30, and 72). A long-standing hypothesis is that HIV exerts its principal cytopathic effect indirectly on uninfected bystander cells rather than directly on infected host cells (30, 72). Cell death was proposed to occur in response to gp120 cross-linking of the CD4 protein on the bystander T cells leading either directly to apoptosis or otherwise priming the cells for apoptosis after T-cell receptor engagement (6, 25, 29, 31, 55). Such a phenomenon would implicate envelope as a central player on the proscenium of HIV type 1 (HIV-1)-induced cell death. The bystander hypothesis was prompted by early data that very few T lymphocytes in the peripheral circulation (on the order of 1/1,000 to 1/8,000) are productively infected during the period of clinical latency after HIV infection (32). Hence, the attrition of CD4+ T cells during the latent period was believed to be due to rare infected cells stimulating uninfected cells to undergo cell death. In studies of this phenomenon, however, the level of apoptosis was variable and affected both CD8 and CD4 cells (6, 20, 30, 55). Moreover, histological analyses of lymph nodes from HIV-infected individuals documented apoptosis in B cells, CD4+ T cells, and CD8+ T cells that appeared to be due to a state of general immune activation not different in character from that observed in uninfected nodes (58). The bystander death hypothesis was also weakened by later estimates of infected cells in blood and lymph node as high as 8 to 16% (24). In recent kinetic models of high cell turnover, a low steady-state level of infected cells during clinical latency is no longer incompatible with a continuous inexorable decline of CD4+ T cells caused by a direct viral cytopathic effect on infected cells (35, 85). Clearly, this is a very important area of study, and it is still under extensive investigation. The focus of this research will be on the mechanism by which HIV-1 kills infected single cells (13).
Several other hypotheses have addressed possible mechanisms of direct viral killing of infected cells. One set of hypotheses suggests that HIV induces apoptosis because fragmented DNA and other potential characteristics of apoptosis can be detected in infected cell populations (27, 34, 46, 71, 78, 92). However, such markers could not be applied quantitatively in these early studies. Attempts to identify the HIV component responsible for apoptosis have failed to reach consensus (17). Apoptosis-inducing effects have been described for env, tat, nef, vpr, and vpu (7, 15, 37, 61, 63, 89). However, much of the evidence on mediators of HIV-1-induced cell death is contradictory. For example, tat has been associated with apoptosis induction in certain studies (7, 49, 66, 70, 87) and as a protection against apoptosis in other studies (28, 54, 91). Evidence for the involvement of the Fas death receptor has been put forward but contested (1, 5, 23, 27, 56, 74, 75, 87, 90). There have also been suggestions that direct virus killing may not involve apoptosis (3, 9, 12, 43, 65, 67). The wide variability of these findings may be due in part to the fact that most studies examined only one or two biochemical features of apoptosis and employed cultures in which the infected and uninfected cells could not be readily distinguished. However, apoptosis and necrosis are known to have cellular, biochemical, and molecular differences (48, 79, 80). Thus, the crucial issue remains whether the death of peripheral blood CD4+ T cells occurred by direct viral killing and whether this death could be ascribed to an apoptotic or necrotic mechanism as previously reported.
One of the most interesting viral components implicated in HIV cytopathicity is the env gene. The involvement of env in cell death was suggested by the observation of mutations confined to the env gene that distinguished cytopathic and noncytopathic derivatives of HIV-1 (14, 26, 45, 81). Other studies suggested that env directly induces a death effect similar to apoptosis after its interaction with the CD4 molecule (19, 21, 38, 40, 44, 51, 52, 62, 73, 82, 87). The implication of env in direct cytopathicity is complicated by the fact that Env is a critical entry molecule for HIV. Thus, env mutations may alter infectivity as well as any subsequent effect of env on cellular physiology. In general, direct viral killing will be diminished if the ability of the virus to propagate is impaired. Therefore, to unambiguously identify a distinct role for env in cell death, it is necessary to establish an infection system in which the virus can productively infect in the absence of env. This can be accomplished by pseudotyping the virus with a highly efficient envelope such as the glycoprotein from vesicular stomatitis virus (VSV-G) (2, 8) so that HIV-1 env is no longer rate-limiting for entry. Such a strategy could be further strengthened by using an HIV mutant that does not encode an entry molecule so that the virus will be limited to a single round of infection. This will eliminate any contribution of env to further rounds of infection that could independently enhance the cytopathic effect on cells in the culture system. Also, this approach should allow an independent assessment of whether superinfection plays any role in the cytopathicity (10, 86). Thus, it is crucial to test the role of env by using HIV-1 that lacks env and depends upon another protein, such as VSV-G, for entry.
Although cytopathicity can encompass many forms of virus-induced cellular dysfunction, in this study we will only examine the death of cells caused by HIV. Cell death, in the broadest terms, has been described by a paradigm involving two general processes: (i) apoptosis that is “programmed” cell death involving a specific molecular pathway and (ii) necrosis or traumatic cell death which may or may not be programmed by a dedicated molecular pathway (79, 80). Apoptosis has been shown to be due to a series of biochemical pathways that are highly conserved in evolution from nematodes to humans. The pivotal event in the apoptosis program is the activation of cysteinyl aspartate-requiring proteases called caspases (60). Activation of these enzymes leads directly to the morphological hallmarks of apoptosis, which include nuclear chromatin condensation and fragmentation, shrinkage and condensation of the cytoplasm with preservation of the plasma membrane integrity, and breakdown of the cell into membrane-enclosed apoptotic “bodies” that are rapidly phagocytosed. Caspase activation also leads to characteristic biochemical changes in the cell, including the inversion of phosphatidylserine to the exterior of the cell membrane and exposure of the mitochondrial antigen recognized by the APO2.7 antibody (42). The biochemical and morphological changes of apoptosis, as well as the ultimate death of the cell can be prevented by soluble inhibitors of caspases (59). In contrast, necrosis is not prevented, and may actually be promoted under certain circumstances, by caspase inhibition (83). Necrotic cells are characterized by the rapid loss of the plasma membrane which leads to swelling, loss of isotonic balance, and massive breakup of the cell components. This is manifested as a complete dissolution of the cell into debris and release of the internal contents. Determining whether apoptosis or necrosis accounts for HIV-induced cell death is a central question in AIDS pathogenesis and should provide insights into the biochemical mechanism by which HIV infection eliminates CD4+ T lymphocytes. We therefore investigated this question in peripheral blood lymphocytes (PBLs) by using both a laboratory-adapted strain and natural isolates of HIV-1. We also assessed the role of the HIV env and nef genes in cytopathicity.
MATERIALS AND METHODS
Cell lines and cell cultures.
Jurkat T lymphoma cells were maintained in RPMI complete medium (RPMI 1640; BioWhittaker) with 10% heat-inactivated fetal calf serum, penicillin (100 U/ml), streptomycin (100 U/ml), l-glutamine (2 mM), and 50 μM β-mercaptoethanol. Jurkat 1.9 was a CD4hi subclone from a low CD4-expressing parental Jurkat line, Jurkat 3 (S. Angleman, D. Bolton, and M. J. Lenardo, unpublished data). Peripheral blood T cells were prepared from anonymous healthy, normal individuals and were obtained from buffy coats collected by heparin-free phlebotomy in the Department of Transfusion Medicine, Clinical Center, National Institutes of Health (NIH). To prepare activated purified CD4+ T cells in early experiments, buffy coats were diluted with phosphate-buffered saline (PBS) 1:5 and underlaid with a 1/3 volume of Ficoll-Paque (Pharmacia). After a 15-min spin (800 × g at room temperature), the cells remaining on the Ficoll cushion were collected and washed three times with PBS. The lymphocytes were then resuspended in complete medium and stimulated with 5 μg of concanavalin A (Boehringer Mannheim) or Phaseolus vulgaris phytohemagglutinin-L (Sigma, St. Louis, Mo.)/ml for 48 to 72 h. Concanavalin A was removed by treatment with 10 mg of α-methyl-mannoside (Sigma)/ml for 30 min at 37°C and three washes with complete medium. CD4+ T cells were obtained by column separation (human T-cell CD4+ subset column kit; R&D Systems) according to the manufacturer's recommendations. Purified CD4+ T cells were then cultured in complete RPMI 1640 with 100 U of interleukin-2 (IL-2 [Proleukin; Midwest Medical, Earth City, Mo.])/ml. PBL cultures were maintained by twice-weekly feeding. In later experiments, buffy coats diluted with PBS were spun for 20 min instead of 15 min. The cells remaining on the Ficoll cushion were then collected and washed once with PBS. They were then resuspended in 10 ml of ACK lysing buffer (Quality Biological, Inc.) for 10 min. After washing them three times with PBS, the cells were resuspended in complete medium and stimulated with 2 μg of phytohemagglutinin-L/ml. After 72 h, the lymphocytes were washed three times in complete RPMI with 100 U of IL-2 (NIH, Frederick, Md.)/ml and stimulated for 24 h in complete medium containing either 100 U of IL-2/ml or a cocktail of 100 U of IL-2, 30 U of IL-12 (PeproTech, Inc.), and 30 U of tumor necrosis factor alpha (TNF-α; PeproTech, Inc.)/ml. Before infection, the lymphocytes were depleted of CD8+ cells by magnetic separation by using fluorescein isothiocyanate anti-human CD8 (PharMingen, San Diego, Calif.) and BioMag Sheep Anti-Fluorescein (Polysciences, Inc.) to prepare CD4-enriched cultures. CD4-enriched cultures were then maintained by daily replenishment of cytokines.
HIV stock and infections.
HIV stocks and plasmids were obtained from the NIAID AIDS Repository unless otherwise indicated. pGFP-HSA, pHIV-EGFP, and pNL-EGFP were obtained from Jacob Reiser at Louisiana State University. The _env_− strain of NL4-3HSA containing a filled-in _Nde_I site creating an early stop codon was a gift of Ned Landau (Salk Institute). HIV-1 (NL4-3HSA strain) stocks were prepared from cell-free supernatant from infected H9 T cells by using an original stock from Ned Landau (Salk Institute) or by plasmid transfection of 293T cells with either the FuGENE reagent (Boehringer Mannheim) or the ExGen 500 reagent (Fermentas) according to the manufacturers' recommendations (18, 33). Mouse heat-stable antigen (HSA) was used as a cell surface marker of provirus expression since it is readily detected by surface staining and is not expressed in human T cells. A large number of control experiments showed that the expression of HSA did not promote or impede the cytopathicity of HIV-1 (data not shown). Primary isolates were passaged no more than once before they were used for infection. VSV-G-pseudotyped viral stocks of NL4-3HSA were harvested 48 h after cotransfection of 293T cells with pLVSV-G or pCMV-VSV-G and pNL4-3HSA env+ or _env_− virus (2, 8, 53, 57). To increase titers in some experiments, virus supernatants (1 ml) were centrifuged at top speed in a microcentrifuge for 1 h, and the bottom 100 μl was used to resuspend the virus on the bottom of the tube and then used for infection. Virus titers were assessed by the MAGI assay by quantitating the number of cells expressing β-galactosidase (69) or by assessing the multiplicity of infection (MOI) by Poisson distribution (11). In early experiments, infections were carried out with a pellet of 0.5 × 106 to 1.0 × 106 CD4+ T cells or Jurkat lymphoma cells in 2063 polypropylene tubes (Falcon) inoculated with 100 μl of HIV-1 (3.8 × 105 infectious units/ml) stock in 200 μl of medium with 1 μg of Polybrene/ml. Samples were centrifuged overnight at 800 × g at room temperature and then resuspended in 5 ml of complete medium in T25 flasks. Cultures were maintained at 5 × 105 to 10 × 105 cells/ml in IL-2 by refeeding as needed. In later experiments, infections were carried out in Biocoat human fibronectin-coated 12-well plates (Becton Dickinson, Franklin Lakes, N.J.). Next, 106 CD4-enriched PBLs or Jurkat lymphoma cells were suspended in complete medium in each well. In PBL infections, the medium was supplemented with 100 U of IL-2 and 5 μg of Polybrene/ml or 100 U of IL-2, 30 U of IL-12, 30 U of TNF-α, and 5 μg of Polybrene/ml. The total volume in each sample well was 3 ml. After the addition of viral stock, the plates were centrifuged at 800 × g for 30 min and then incubated at 37°C. Cultures were maintained by daily cytokine feeding. In experiments with indinavir (IND; AIDS Repository), a 10 μM concentration was added to the appropriate samples prior to centrifugation, after centrifugation, and daily throughout the course of the infection.
Assays for cell viability and quantitation of infection.
HIV-1 cytopathicity and cell death was assessed by flow cytometric forward scatter-side scatter (FSC-SSC) profiles obtained on a FACSCalibur flow cytometer (Becton Dickinson) and fluorescent profiles of cells stained with 1:200-diluted anti-mouse HSA phycoerythrin (PE) (CD24; PharMingen) daily throughout the course of infection. Quantitation of the level of infection was also carried out with intracellular HIV-1 p24 staining. Cells were fixed by using the Cytofix/Cytoperm kit (PharMingen) according to manufacturer's instructions and then stained with an appropriate dilution of anti-p24 PE antibody, KC57-RD1 (Coulter), at 4°C for 30 min, washed twice, and analyzed by flow cytometry. Heat-shocked necrotic control samples were prepared by heating at 56°C for 10 to 30 min. Flow cytometry results were analyzed by FlowJo (Tree Star, Inc., San Carlos, Calif.) or CellQuest software (Becton Dickinson). Comparison of infected-cell death by using env+ versus _env_− strains of NL4-3HSA in CD4-enriched PBLs was performed by first gating on the HSA-positive population and then determining the fraction of viable cells within this population.
Apoptosis assays.
Infected and mock-infected CD4+ T cells or Jurkat cells were treated with 1 μg of staurosporine (Alexis Biochemicals, San Diego, Calif.)/ml for various times. Annexin V binding was used to measure phosphatidylserine exposure on the outer leaflet of the plasma membrane by incubating 106 cells in 1:30-diluted fluoresceinated Annexin V (PharMingen) in Annexin V binding buffer (10 mM HEPES-NaOH, pH 7.4; 150 mM NaCl; 5 mM KCl; 1 mM MgCl2; 2 mM CaCl2) for 15 min. Cells were washed and analyzed by flow cytometry. Exposure of the 7A6 mitochondrial membrane antigen was measured by using the APO2.7 antibody labeled with PE (Immunotech/Coulter, Marseilles, France) according to the manufacturer's instructions. Briefly, 106 cells were permeabilized in 100 μg of digitonin/ml in PBSF buffer (PBS with 2.5% [vol/vol] heat-inactivated FCS and 0.1% NaN3) for 20 min on ice, washed, incubated in 1:5 diluted APO2.7-PE for 15 min at room temperature, and washed again prior to analysis on a FACSCalibur cytometer. To exclude nonspecific binding of the staining reagents to dead or necrotic cells, all quantitative flow cytometric analyses were gated on a “viable” FSC-SSC population such that the data reflect only cells that are viable or recently committed to undergo apoptosis.
Microscopic analysis of cell death.
Transmission electron microscopy (TEM) was performed on suspensions of cells fixed in neutral buffered glutaraldehyde and gelled in agarose. Postfixation was carried out with osmium tetroxide, followed by dehydration in graded ethanol and propylene oxide and embedding in Spurr's epoxy. One-micron semithin plastic sections were cut and stained with the combined methylene blue, Azure II, basic fuschin stain for light microscope selection of blocks to be thinned for TEM. Thin sections were stained with uranyl acetate and lead citrate and viewed on a LEO EM10 transmission electron microscope at 60 kV. The number of apoptotic, necrotic, and normal cells was assessed by inspection by an evaluator unaware of the identity of the samples. Controls of necrotic or apoptotic cells, generated by heat-shock or staurosporine treatment, respectively, were used as the basis of characteristic morphology.
RESULTS
HIV-1-induced cytopathicity can be quantitatively attributed to necrosis and not apoptosis.
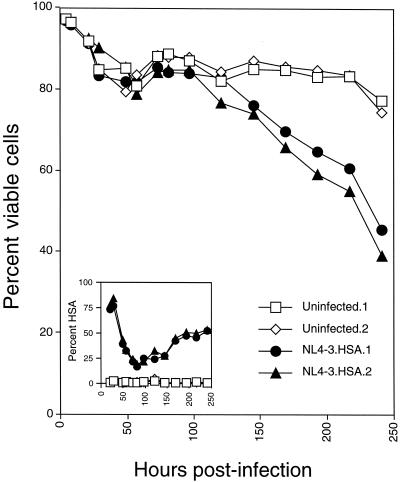
To establish an in vitro system, we infected cultures of activated purified CD4+ T lymphocytes with the NL4-3HSA strain of HIV-1 that harbors the coding sequence of the mouse HSA in place of the nef gene (18, 33). This allowed us to clearly distinguish infected from uninfected cells by flow cytometry and examine the cytopathic effect on each one independently. This technique has been used successfully by other groups (17, 32). When we infected CD4+ T lymphocytes with HIV-1, we observed a progressive loss of viability that was initiated at about day 4 and continued over 10 days of infection (Fig. 1). This loss of viability was only observed in cultures containing cells expressing the HSA marker (Fig. 1, inset). In these experiments, we consistently observed an initial high level of HSA that reached a nadir at ca. 75 h and then increased until the end of the experiment. Biphasic expression of HSA was due to the initial passive transfer of the protein from virus fusion, followed by a second wave of expression (starting at 75 h in these culture conditions) from the integrated provirus (D. Bolton, B.-I. Hahn, and M. J. Lenardo, unpublished results). Thus, a lethal cytopathic effect was readily observed under our culture conditions that correlated with expression of the HIV genome. We observed essentially no death of uninfected bystander cells or even cells with low-level provirus expression (see below). It was notable that early in infection (within the first 2 days), there was a loss of viability of <20% evident in the uninfected (mock) and infected cultures alike. Thus, despite the high level of virus-cell interactions, as indicated by the high level of HSA passively acquired by the virion-cell fusion in ca. 80% of the cells, there was no death triggered. This suggests that the previously proposed mechanisms for T-cell death in response to extensive gp120-CD4 interactions do not appear to account for the cytopathic effect we observed (6, 81).
FIG. 1.
HIV-1 causes a profound cytopathic effect on cultured CD4+ T lymphocytes from peripheral blood. Purified CD4+ T lymphocytes were activated with concanavalin A and IL-2 and then infected with the NL4-3HSA strain of HIV-1. Duplicate uninfected or infected cultures were analyzed by flow cytometry for the fraction of viable cells and the expression of HSA as indicated (inset). Viable cells were determined by forward scatter versus side scatter. Infected cells are detected from cells in the viable gate. Note that early HSA is “donated” to the target cells by the virions that have acquired this membrane protein from the producer cells prior to 50 h in this experiment, whereas later HSA is due to provirus expression (11).
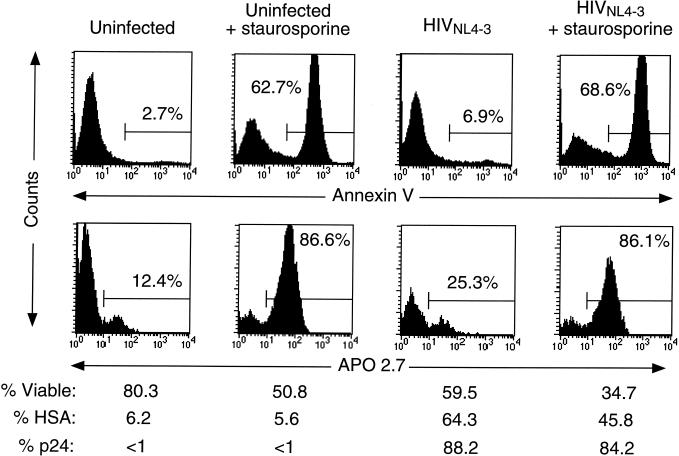
In a separate study of the cytopathic effect of HIV-1 on T-cell tumor lines, we observed that there was no consistent display of apoptosis markers and that virally induced cell death was not dependent upon caspase activation (11). We therefore examined two early hallmarks of apoptosis in infected peripheral blood CD4+ T cells: Annexin V, which binds to cells that externally display phosphatidylserine, and the exposure of the mitochondrial antigen recognized by APO2.7 (Fig. 2). We found that in highly infected CD4+ T-cell cultures in which the loss of viable cells was substantial, only modest levels of phosphatidylserine exposure (top panels) or APO2.7 (bottom panels) were observed even when a striking cell loss was observed. In contrast, dramatic increases in these markers were seen when the apoptosis inducer staurosporine was added to either infected or uninfected cultures. Similar results were obtained with an agonistic anti-Fas antibody (data not shown). We also observed similar results with Annexin V and APO2.7 in tumor cell lines (11).
FIG. 2.
HIV-associated cell death does not correlate with externalization of phosphatidylserine or exposure of the mitochondrial antigen recognized by the monoclonal antibody APO2.7. Purified CD4+ T cells were infected with HIV-1 (NL4-3HSA) for 8 days or mock infected. On the ninth day, both uninfected and infected cultures were treated with staurosporine (1 μg/ml) for 7 h. The percent of viable cells was determined by FSC-SSC plots; the fraction of infected cells was measured by surface staining for HSA and, more sensitively, by internal staining for the p24 antigen. Note the lower fraction of HSA exhibited on the surface of the staurosporine-treated cells, indicating augmentation of cell death. Each panel represents 10,000 events in the live FSC-SSC gate.
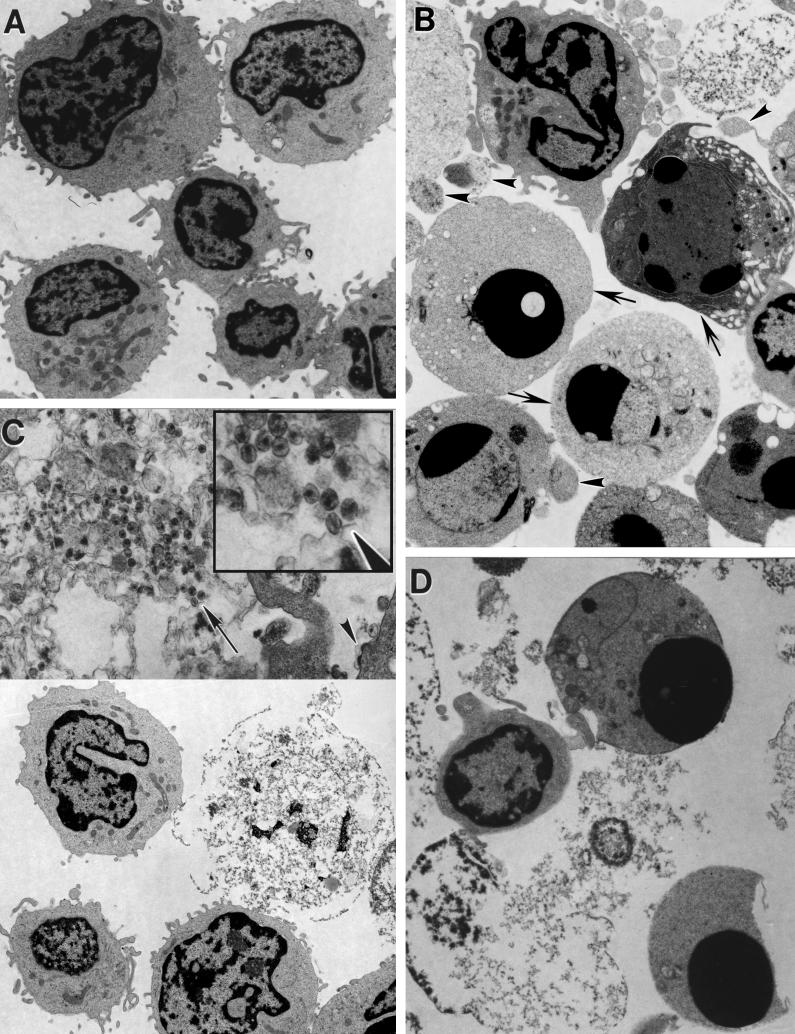
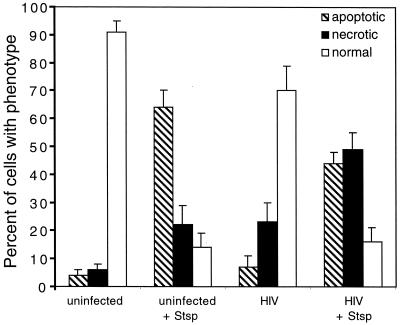
The difference between apoptosis and necrosis was originally based on morphological distinctions, and one of the best tools for characterizing these modes of death remains morphological analysis such as those done by TEM (Fig. 3) (88). We observed that there was little apoptosis visible in the uninfected (Fig. 3A) or infected samples (Fig. 3C). Rather, in the HIV-infected cultures, we observed mostly highly fragmented cells that were swollen and had lost integrity of the plasma membrane, resembling a necrotic form of cell death (41) (panels C and D). In some of the corpses of infected cells, mature virus particles could be seen within the necrotic debris (Fig. 3C, inset, arrowhead). In contrast, treatment of the samples for several hours with staurosporine caused the emergence of a clear apoptosis phenotype in uninfected controls or virus-infected samples (panels B and D). Apoptotic features included the compaction and margination of chromatin to form pyknotic nuclei, condensed but otherwise intact cytoplasm, and the formation of membrane-enclosed apoptotic bodies (Fig. 3B, arrowheads). These characteristics differed dramatically from normal cells, which remained intact and exhibited open reticular chromatin (Fig. 3B, arrows). Although not entirely absent, apoptotic features were not prominent in a large variety of samples of CD4+ T cells undergoing death after HIV infection. To quantify these observations, we photographed a number of sections at low magnification and scored a large number of individual cells for apoptotic, necrotic, or normal morphology (Fig. 4). These data revealed that a preponderance of apoptotic cells was only seen upon staurosporine treatment in either uninfected or infected cells. In contrast, virus infection dramatically increased the number of necrotic cells but not the number of apoptotic cells. Notably, staurosporine also significantly increased the number of necrotic cells in both uninfected and infected cultures. This likely results from apoptotic cells undergoing secondary necrosis, since there is no phagocytic removal of the apoptotic cells in this culture system (36). In addition, staurosporine and other apoptosis inducers may exacerbate necrosis induced by viral infection, as has been observed with anti-Fas treatment of HIV-infected Jurkat cells (11). Taken together, these data strongly suggest that necrosis quantitatively accounted for the majority of cytopathic death due to infection of CD4+ T cells with the NL4-3HSA virus.
FIG. 3.
Electron micrographs of HIV-1-infected CD4+ T lymphocytes compared with staurosporine-induced apoptosis. CD4+ T lymphocytes derived from peripheral blood were activated, either infected with NL4-3HSA _env_− virus for 9 days or mock infected, and on the tenth day treated with staurosporine (1 μg/ml) for 4 h. Cells were then embedded in epoxy resin and examined by TEM. Samples include mock-infected cells (×4,900) (A), mock-infected cells with staurosporine added (×5,400) (B), infected cells (×4,900 [lower half], ×17,000 [upper half]) (C), and infected cells with staurosporine added (×7,200) (D). In panel B, the arrows indicate cells with compacted chromatin, and the arrowheads indicate apoptotic bodies. In panel C, the arrowhead indicates budding virions. The inset in panel C represents a 2.2-fold magnification of the region indicated by the arrow and illustrates the finding of mature retroviral particles within the debris of a necrotic cell.
FIG. 4.
Quantitation of the level of apoptosis and necrosis in CD4+ T lymphocytes derived from peripheral blood that were either infected with NL4-3HSA for 10 days or mock infected and then treated with staurosporine (Stsp; 1 μg/ml) for 7 h. The samples were embedded in epoxy resin, sectioned, and stained with blue stain. The fractions of cells with apoptotic, necrotic, and normal appearances in the light microscope were quantified by an examiner who was blinded to the origin of the samples. The numbers in each cell sample total 100% except for slight rounding errors. Approximately 200 to 500 events were tabulated for each sample.
Natural isolates of HIV-1 also manifest a necrotic cytopathicity.
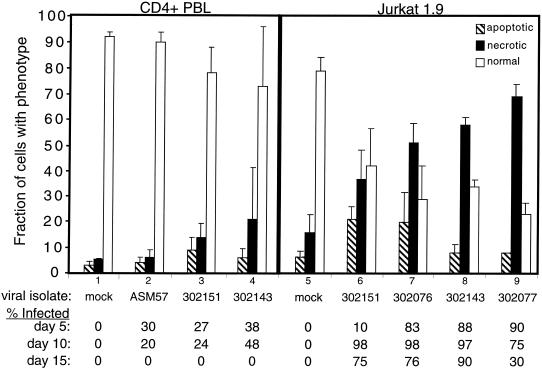
To determine whether these results were also characteristic of natural infections, we examined infections of both peripheral CD4+ T lymphocytes and Jurkat 1.9 T cells with primary isolates of HIV-1. After the initial infection, we carried out flow cytometry on the culture to determine the level of cell viability and fraction of provirus-expressing cells, as indicated by intracytoplasmic staining for p24. At day 11, we harvested the culture and carried out microscopic analyses to determine the number of cells manifesting an apoptotic, necrotic, or normal morphology. The results of the microscopic analysis were then compared to the level of infection at days 5, 10, and 15 (Fig. 5). We found that cell death was positively correlated with isolates that spread rapidly and infected most of the cells in the culture. Also, the loss of normal viable cells was more dramatic in Jurkat cells (right panel) in which the virus infection was greater than in peripheral CD4+ T cells (left panel). The great majority of dead cells observed with all isolates mostly manifested a necrotic phenotype, and only a minor fraction of apoptotic cells were seen. In the control infections of CD4+ T cells and Jurkat cells, cultures 1 and 5, respectively, the cultures contained primarily normal cells with only a small number of adventitious apoptotic or necrotic cells. In the case of the infections of CD4+ T cells, we found that the cultures exhibited low levels of infection that peaked on day 10 and were extinguished by day 15. None of the primary isolates gave strong infections and the infection was lost after 2 weeks due to the death of infected cells. Infections with the primary isolates using Jurkat 1.9 T cells (that had been selected for high expression of CD4) gave a much greater fraction of infected cells, and this occurred with more rapid kinetics, with the most robust infection peaking as early as day 5. As a consequence, in each of the infected Jurkat cultures there was florid necrotic cell death with a large loss of healthy cells compared to the uninfected control. We conclude that cytopathicity is associated with primary isolates of HIV-1 and correlates with the level of infection; moreover, the cytopathic component of the virus induces cell death by necrosis rather than apoptosis.
FIG. 5.
Analysis of the death effect on peripheral blood CD4+ T lymphocytes and Jurkat 1.9 T cells infected with primary isolates of HIV-1 in vitro. The samples were prepared and analyzed as in Fig. 4. Cultures 1 to 4 represent CD4+ T cells; cultures 5 to 9 represent Jurkat cells. The isolates used in each infection were as follows: cultures 1 and 5, mock-infected control; culture 2, ASM 57 virus from a long-term nonprogressor; cultures 3 and 6, dualtropic virus 302151; cultures 4 and 8, dualtropic virus 302143; culture 7, dualtropic virus 302076; and culture 9, dualtropic virus 302077. Note that the greater infectivity of Jurkat cells compared to CD4+ T cells was evident in cultures 3 and 6 as well as cultures 4 and 8, since each pair of cultures used a common primary isolate. At the bottom is provided the fraction of cells expressing p24 measured by intracytoplasmic staining and flow cytometry for each of the indicated time points. A portion of the culture was harvested for microscopy on day 11. On average, 100 to 150 cells for each culture were evaluated for morphological features of apoptosis or necrosis from photographs of stained microscope slides by a blinded observer.
Env is not required for HIV-1-induced death of Jurkat cells.
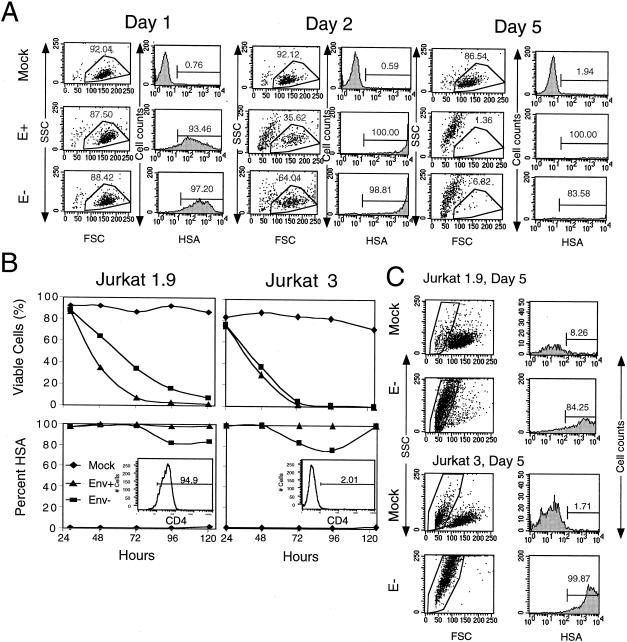
To investigate the role of the envelope in HIV-1-induced cytopathic death, we compared NL4-3HSA with an intact env gene to an Env-deficient version of NL4-3HSA containing an early stop codon in the env gene. We pseudotyped the virions for both genomes with the VSV-G protein to ensure that the HIV-1 envelope was not rate limiting for virus entry. This allowed us to test whether Env had a direct toxic effect in the infected target cells independent of its role in virus entry. We infected Jurkat 1.9 T cells with either HIV-1 NL4-3HSA env+ or _env_− strains at a comparable MOI as determined by the MAGI assay (Fig. 6A and B [left panel]). Within 5 days, essentially all of the infected cells had died, independent of Env expression. However, the presence of Env clearly provided an acceleration of the rate of death. At day 1 postinfection, we found that the cells infected with either env+ or _env_− virus were highly infected, with similar viability. By day 2, only 35% of cells infected with env+ virus remained viable, whereas cells infected with _env_− virus were still 64% viable. Ultimately, all viability was lost by day 3 in the env+ culture compared to day 5 in the _env_− culture (Fig. 6A, right panels, and data not shown). The mock-infected cells remained >86% viable throughout the experiment. We verified through other experiments (data not shown) that the env+ virus caused a loss of viability ca. 1 to 2 days sooner than the _env_− virus. Moreover, we could not attribute the death induced by the _env_− NL4-3HSAvirus to the VSV-G coat since pseudotypes with Mokola virus, pseudorabies virus, or amphotropic murine leukemia virus coats also mediated cytopathic infections with the _env_− NL4-3HSA genome (data not shown). It was also notable that the cells in the “dead” gate for infected cultures in these experiments contained only highly infected cells, indicating that only infected cells and not bystander cells were killed (Fig. 6C). Thus, Env appears not to be required for direct HIV-1-induced cytopathic death in infected cells.
FIG. 6.
NL4-3 strains lacking the envelope gene retain their cytopathic effect. (A) Dot plot and histograms exhibiting the fraction of viable cells by FSC-SSC profiles (left panels) and the fraction of infected Jurkat 1.9 cells by HSA at days 1, 2, and 5 contained within the live gate for cultures mock infected, infected with NL4-3HSA env + virus (E+), or infected with NL4-3HSA env − virus (E−) at an MOI of 10 (right panels). The numbers indicate the percentage of cells within the gates shown. (B) Quantitation of the fraction of viable cells (top panels) and the fraction of infected cells indicated by HSA (bottom panels) as a function of time for cultures of mock infected (⧫), NL4-3HSA env + virus infected (▴), or NL4-3HSA env − virus infected (▪) in independent experiments with either Jurkat 1.9 or Jurkat 3 cells as indicated. Insets show the level of CD4 on the Jurkat sublines used. (C) Dot plot and histograms of mock and E− infections exhibiting a gate of nonviable cells by FSC-SSC profiles (left panels) that were then analyzed for the percentage of infected Jurkat 1.9 cells or Jurkat 3 cells by HSA at day 5. The numbers indicate the fraction of the cells within the gates shown. The results are representative of 20 experiments.
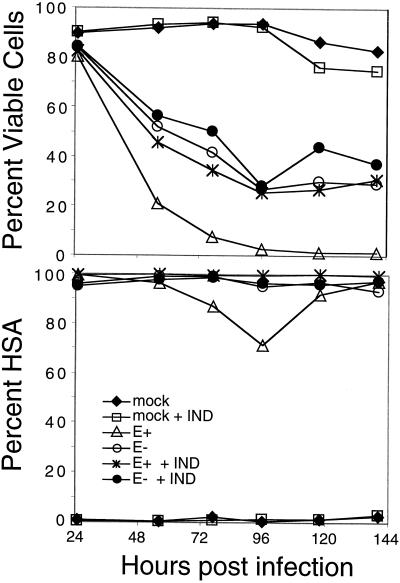
In an independent experiment, we found that the disparity between NL4-3HSA env+ and _env_− HIV-1 was eliminated when a CD4lo cell line, Jurkat 3, was infected, indicating CD4 dependence (Fig. 6B, right panel). Note that in this experiment, more virus was used so that the kinetics of death for both env+ virus and _env_− was accelerated (Fig. 6B, compare left and right panels). Therefore, the difference could be due to the ability of the env+ strain of HIV-1 to generate infectious virions that could reinfect cells in the culture, implying that Env could have its effect solely as a viral entry function. Consistent with this interpretation, we found that env+ virus gave significantly higher overall virus expression, as indicated by the cell of HSA on infected cells, than the _env_− virus (compare HSA levels on day 1 and day 2 for E+ and E− in panel A). Also, the NL4-3HSA virus lacks nef and therefore causes a slower and less-complete downmodulation of CD4, thereby permitting reinfection (data not shown). To investigate this possibility, we used the protease inhibitor, IND, to prevent the maturation of noninfectious to infectious viral particles. One day after infection all of the samples were already highly infected, although they showed similar viability (Fig. 7). By day two, the env+ virus-infected cells died at a much faster rate than those infected with the _env_− virus. However, the addition of IND to the env+ culture completely eliminated this advantage over the _env_− culture. A similar trend continued throughout the course of the infection. Cultures in which IND was added to Env− infections or uninfected cells showed no effect of the drug on cell viability. Lastly, further control experiments in which we introduced the plasmids containing the HIV-1 NL4-3HSA Env+ or Env− genomes into the Jurkat 1.9 T cells by electroporation revealed a greater loss of viability when Env was present and promoted reinfection (data not shown). Taken together, these results lead to the important conclusion that Env is not required for a direct cytopathic effect but rather promotes cytopathicity by increasing the level of virus expression by facilitating viral entry.
FIG. 7.
Accelerated death rate of HIV-1 NL4-3HSA env + virus-infected cells is due primarily to reinfection and is abrogated by the protease inhibitor IND. Jurkat 1.9 T cells were infected at an MOI of 1 by either NL4-3HSA env + virus or the NL4-3HSA env − virus, and 10 μM IND was added to the samples as indicated. IND was replenished daily. “Mock” indicates that no virus was added. Quantitation of the fraction of viable cells and infected cells is plotted as a function of time as in Fig. 6. Results are representative of five experiments.
HIV-1 induces death of CD4-enriched PBLs in the absence of Env.
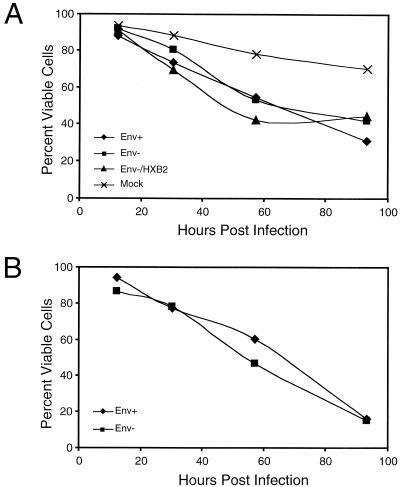
We further tested the physiological relevance of our observations of Env as a viral entry factor, but not cytopathic factor, by comparing infections of CD4-enriched PBLs with the env+ and env− NL4-3HSA mutants pseudotyped with VSV-G protein (Fig. 8A). We observed similar levels of death in PBLs highly infected with either the env+ NL4-3HSA or the env− NL4-3HSA mutant when HSA expression was matched between samples. As a further control, we also used HIV-1 NL4-3HSA env mutant pseudotyped with both VSV-G and an HXB2 HIV-1 envelope and found no significant increase in cytopathicity. Furthermore, when we gated on the most highly infected cells over the course of the infections as indicated by HSA expression, we found a striking loss of viability for both the env+ and env− viral stocks (Fig. 8B). Hence, the dramatic cytopathic effect observed in this in vitro system was not dependent on the presence of HIV-1 Env.
FIG. 8.
PBLs infected with either HIV-1 NL4-3HSA env + or env− strains show comparable cytopathic effects. (A) Viable cells quantitated by FSC-SSC as in Fig. 6. Samples were infected with NL4-3HSA env+ HIV-1, NL4-3HSA _env_− HIV-1, or NL4-3HSA env − HIV-1 pseudotyped with the HXB2 HIV-1 envelope or were mock infected as indicated; all HIV-1 NL4-3HSA strains used in these experiments were also pseudotyped with VSV-G protein. (B) Percent viability of highly infected PBLs as determined by high HSA expression (fluorescence intensity above the second decade). Samples were infected with HIV-1 NL4-3HSA env + virus and HIV-1 NL4-3HSA env − virus pseudotyped with VSV-G as indicated.
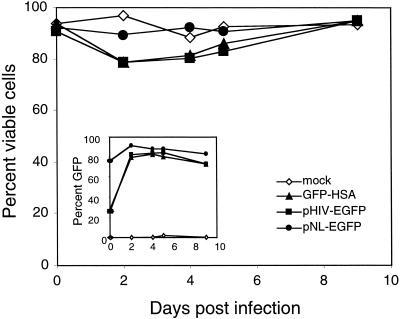
To rule out spurious toxicity of the VSV-G protein and to determine whether any viral protein of HIV-1 was necessary for the cytopathic death, Jurkat 1.9 T cells were infected with three different NL4-3 derivatives in which green fluorescent protein (GFP) replaced the natural viral proteins (16, 57). We found no appreciable loss of viability (Fig. 9). All of the genomes were unable to express any of the normal HIV-1 viral proteins, with the exception of pHIV-EGFP, which expresses the viral regulatory proteins, Tat, Vpu, and Rev. GFP expression was detected in the viable cell population (Fig. 9, inset), indicating that the cells were highly and comparably infected but exhibited none of the cytopathic effects observed in cells infected with wild-type and env− virus (Fig. 6 and 7 and data not shown). Therefore, HIV-1 protein(s) expressed from the provirus, other than Env, are necessary for the observed cytopathic effect of HIV-1 in the infected T cells in this culture system.
FIG. 9.
NL4-3 derivatives devoid of natural viral protein-coding sequences are not cytopathic. Three NL4-3 HIV-1 derivatives—HIV-GFP-HSA, pHIV-EGFP, and pNL-EGFP—that are unable to express any of the viral proteins (except for pHIV-EGFP, which expresses Tat and Rev) were used to infect Jurkat 1.9 T cells and then monitored for 9 days. The fraction of viable cells postinfection was assessed by flow cytometry as in Fig. 6, and GFP expression was examined in the viable cell population (inset). A total of 10,000 events were collected for each datum point.
DISCUSSION
How are CD4+ T cells depleted? This question is central to our understanding of the pathogenesis of HIV-1 infections. A great deal of experimental work has been directed at this question, and a plethora of different models have emerged (4, 72, 73). As better techniques have become available, it has been possible to address this issue with greater precision. It is clear from our studies that HIV-1 is a highly cytopathic virus for its natural host, CD4+ T lymphocytes. In well-controlled studies of CD4+ T lymphocytes in vitro in which infected and uninfected cells could be readily distinguished by flow cytometry, we found no evidence of bystander killing, a finding that is in agreement with previous in vitro studies (27). Thus, our efforts focused on how HIV-1 causes a direct cytopathic effect on single infected cells, as emphasized previously by Sodroski and coworkers (13). If bystander death has an important impact in vivo, then it may result from the general state of immune activation or involve other factors that are not present in our in vitro culture system (58). Although many studies suggest that apoptosis is responsible for CD4+ T-lymphocyte death, we could not find evidence to support this contention. Rather, as previously suggested, we find that the T-cell death induced by both laboratory-adapted strains and natural isolates had hallmarks of necrosis rather than apoptosis (43, 65). This was especially evident from TEM studies which showed that HIV-1 caused complete dissolution of the cell without the classic features of apoptosis. This finding was surprising to us because of the number of studies that document apoptotic changes in HIV-1-infected cultures. The previous findings may be due, at least in part, to the fact that HIV-1 infection damages the cell and makes it more susceptible to apoptosis induced by other agents under some conditions (11, 47, 50, 64). It therefore becomes crucial to demonstrate that the apoptosis observed quantitatively accounts for the loss of viable cells due to infection. Such evidence has not been previously reported, and our data now indicate that necrosis rather than apoptosis quantitatively accounts for the level of cytopathic death which directly relates to the level of HIV-1 infection in T-cell cultures.
The predominance of “necrotic” death is likely to be relevant in vivo since the same results were obtained with laboratory-adapted HIV-1 strains, as well as natural isolates, in both Jurkat T lymphoma cells and CD4+ T lymphocytes. However, since precise molecular mechanisms of necrotic death have not been defined, terming the death “necrosis” becomes a diagnosis of exclusion for this type of viral cytopathicity. Nonetheless, HIV-induced death is not programmed in the sense of triggering death through caspases since features of this type of death were lacking. Thus, caspase inhibitors would not be therapeutically useful for preventing T-cell loss in HIV-1 infection and could even be harmful since they can promote necrosis under certain conditions (83). Rather, HIV-1 inflicts trauma on the cells that apparently causes a vital component of the cell to fail, thereby leading to dissolution of the cells. Identifying the virus function that mediates cellular damage will be crucial for understanding this event.
A prominent candidate for the mediator of cell death has been considered to be the Env protein. A variety of previous studies have provided evidence of its participation in the death process mainly by showing that alterations of the env gene diminish the cytopathicity of HIV-1 (14, 26, 45, 81). However, the interpretation of these experiments hinges critically on eliminating any effect that env alterations could have on the process of infection. For example, subtle changes in the envelope protein that affect viral entry might decrease the efficiency of viral spreading throughout the culture, resulting in less observed death. We sought to avoid this complication by employing the highly efficient VSV-G coat protein in an infection procedure in which essentially all cells are infected in a single step. Under these conditions, HIV-1 Env is not rate limiting for initial virus entry. The efficiency of viral fusion and, ultimately, productive infection were readily assessed on a cell-by-cell basis by flow cytometric analysis of both surface HSA encoded in place of nef and intracytoplasmic staining for p24. In this system, there were apparently no direct toxic effects of Env-CD4 interactions, which have been previously implicated in uninfected bystander cell death (72, 73). Since we use a high MOI, this type of indirect killing would likely occur during or immediately after adsorption, and yet there was no significant early mortality. Most importantly, cell killing did not differ between env+ and env− viruses. Therefore, although gp160 can apparently induce apoptosis and have other injurious effects on T cells in certain contexts, under conditions of actual virus infection in our culture system, it was clearly dispensable.
The involvement of superinfection or other envelope-receptor interactions have been proposed as essential mechanisms of cytopathicity for both human and nonhuman retroviruses (73, 77). Comparing the pseudotyped env+ and env− viruses provided a well-controlled test of whether the Env protein has a directly cytopathic effect in the infected cells. Previous work used env mutants that were not totally innocuous in their effects on infectivity, and the effect of pseudotyping was not tested (14, 26, 45, 81). Our data show that the level of infection is an important determinant of the degree of cytopathicity (Fig. 1 and 5). Hence, even subtle effects on the infectivity of the virions could have indirect confounding effects on the level of cell death. In fact, this may be the major explanation for why previous analyses that have implicated Env in the cytopathic effect differ from our results. In the present experiments, the rate-limiting step to entry is no longer the HIV envelope, since we obtained high-titer pseudotyped viral stocks irrespective of whether the env gene was mutated or intact. Also, since the VSV-G coat was supplied in trans (as opposed to being encoded in the viral genome), the infection was one round for the env− virus stocks. Hence, the effects on cytopathicity could only have been due to provirally expressed proteins in cells that had been infected during a single round. Single-round infection with the env− virus also provided a convincing approach to addressing the potential involvement of superinfection or syncytia to the HIV-1 death effect. We found that only viruses with intact env could form syncytia, which was most apparent when infecting adherent cells such as MAGI cells (data not shown). The env− virus also failed to cause reinfection after the first round, although the pseudotyped virus with intact env was capable of additional rounds of infection. The inability of the env− virus to cause syncytia or carry out multiple rounds of infection and superinfection did not prevent its cytopathic effect. Thus, the evidence is compelling that superinfection, reinfection, or syncytium formation is not necessary for the cytopathic effect of HIV-1 in this in vitro culture system, although reinfection hastens cellular demise. It remains to be seen whether other roles for env in cytopathicity may emerge as important for pathogenesis in whole-animal infections.
Another point that our experiments address is whether nef is directly involved in T-cell killing (39). Nef is an enigmatic protein that may have several functions in the life cycle of primate lentiviruses (76). In our experiment, the HSA gene replaced the nef coding sequence in the NL4-3HSA virus so there was no expression of the Nef protein. Both infectivity and cytopathicity were not substantially different from other HIV-1 strains that had an intact nef gene (data not shown). Thus, we could find no evidence for a requirement for Nef in cytopathicity. Nevertheless, replacing all HIV-encoded native viral functions with GFP rendered the viral genome noncytopathic, indicating that HIV encodes a cytopathic function. Since these GFP-bearing HIV strains were also VSV-G pseudotyped, this would eliminate any possibility that the VSV-G coat protein accounted for the cytopathic effects of the env+ or env− NL4-3HSA viruses. Taken together, the results suggest that one or more virus function(s) other than the env or nef are essential for a direct nonapoptotic cytopathic death of cultured CD4+ T lymphocytes caused by HIV-1.
Acknowledgments
We thank Ned Landau, Theresa Gurney, Fabio Candotti, and Jacob Reiser for plasmids, cells, and advice during the initiation of this project. We thank Anthony Fauci and members of his laboratory for use of P3 research facilities and for helpful advice and encouragement. We are also grateful to John Coffin, Eric Freed, Steve Hughes, and Malcolm Martin for advice and assistance; Francis Chan, Richard Siegel, Hyung Chun, and Lixin Zheng for helpful insights and inspiring discussions; and Keiko Sakai for a critical reading of the manuscript.
D.L.B. was a participant in the FAES (NIH)-Johns Hopkins University Cooperative Graduate Program in Biomedical Sciences.
REFERENCES
- 1.Accornero, P., M. Radrizzani, D. Delia, F. Gerosa, R. Kurrle, and M. P. Colombo. 1997. Differential susceptibility to HIV-GP120-sensitized apoptosis in CD4+ T-cell clones with different T-helper phenotypes: role of CD95/CD95L interactions. Blood 89**:**558-569. [PubMed] [Google Scholar]
- 2.Aiken, C. 1997. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 71**:**5871-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antoni, B. A., P. Sabbatini, A. B. Rabson, and E. White. 1995. Inhibition of apoptosis in human immunodeficiency virus-infected cells enhances virus production and facilitates persistent infection. J. Virol. 69**:**2384-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badley, A. D., D. Dockrell, and C. V. Paya. 1997. Apoptosis in AIDS. Adv. Pharmacol. 41**:**271-294. [DOI] [PubMed] [Google Scholar]
- 5.Badley, A. D., J. A. McElhinny, P. J. Leibson, D. H. Lynch, M. R. Alderson, and C. V. Paya. 1996. Upregulation of Fas ligand expression by human immunodeficiency virus in human macrophages mediates apoptosis of uninfected T lymphocytes. J. Virol. 70**:**199-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banda, N. K., J. Bernier, D. K. Kurahara, R. Kurrle, N. Haigwood, R. P. Sekaly, and T. H. Finkel. 1992. Cross-linking CD4 by human immunodeficiency virus gp120 primes T cells for activation-induced apoptosis. J. Exp. Med. 176**:**1099-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartz, S. R., and M. Emerman. 1999. Human immunodeficiency virus type 1 Tat induces apoptosis and increases sensitivity to apoptotic signals by upregulating FLICE/caspase-8. J. Virol. 73**:**1956-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartz, S. R., and M. A. Vodicka. 1997. Production of high-titer human immunodeficiency virus type 1 pseudotyped with vesicular stomatitis virus glycoprotein. Methods 12**:**337-342. [DOI] [PubMed] [Google Scholar]
- 9.Bergamini, A., L. Dini, M. Capozzi, L. Ghibelli, R. Placido, E. Faggioli, A. Salanitro, E. Buonanno, L. Cappannoli, L. Ventura, M. Cepparulo, L. Falasca, and G. Rocchi. 1996. Human immunodeficiency virus-induced cell death in cytokine-treated macrophages can be prevented by compounds that inhibit late stages of viral replication. J. Infect. Dis. 173**:**1367-1378. [DOI] [PubMed] [Google Scholar]
- 10.Bergeron, L., and J. Sodroski. 1992. Dissociation of unintegrated viral DNA accumulation from single-cell lysis induced by human immunodeficiency virus type 1. J. Virol. 66**:**5777-5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolton, D. L., B.-I. Hahn, E. A. Park, L. L. Lehnhoff, F. Hornung, and M. J. Lenardo. 2002. Death of CD4+ T-cell lines caused by human immunodeficiency virus type 1 does not depend on caspases or apoptosis. J. Virol. 76**:**5094-5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borthwick, N. J., R. G. Wickremasinghe, J. Lewin, L. D. Fairbanks, and M. Bofill. 1999. Activation-associated necrosis in human immunodeficiency virus infection. J. Infect. Dis. 179**:**352-360. [DOI] [PubMed] [Google Scholar]
- 13.Cao, J., I. W. Park, A. Cooper, and J. Sodroski. 1996. Molecular determinants of acute single-cell lysis by human immunodeficiency virus type 1. J. Virol. 70**:**1340-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao, J., B. Vasir, and J. G. Sodroski. 1994. Changes in the cytopathic effects of human immunodeficiency virus type 1 associated with a single amino acid alteration in the ectodomain of the gp41 transmembrane glycoprotein. J. Virol. 68**:**4662-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casella, C. R., E. L. Rapaport, and T. H. Finkel. 1999. Vpu increases susceptibility of human immunodeficiency virus type 1-infected cells to fas killing. J. Virol. 73**:**92-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chinnasamy, D., N. Chinnasamy, M. J. Enriquez, M. Otsu, R. A. Morgan, and F. Candotti. 2000. Lentiviral-mediated gene transfer into human lymphocytes: role of HIV-1 accessory proteins. Blood 96**:**1309-1316. [PubMed] [Google Scholar]
- 17.Cohen, J. 1995. Researchers air alternative views on how HIV kills cells. Science 269**:**1044-1045. [DOI] [PubMed] [Google Scholar]
- 18.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206**:**935-944. [DOI] [PubMed] [Google Scholar]
- 19.Corbeil, J., and D. D. Richman. 1995. Productive infection and subsequent interaction of CD4-gp120 at the cellular membrane is required for HIV-induced apoptosis of CD4+ T cells. J. Gen. Virol. 76**:**681-690. [DOI] [PubMed] [Google Scholar]
- 20.Cotton, M. F., C. Cassella, E. L. Rapaport, P. O. Tseng, S. Marschner, and T. H. Finkel. 1996. Apoptosis in HIV-1 infection. Behring Inst. Mitt. 97**:**220-231. [PubMed] [Google Scholar]
- 21.Cottrez, F., F. Manca, A. G. Dalgleish, F. Arenzana-Seisdedos, A. Capron, and H. Groux. 1997. Priming of human CD4+ antigen-specific T cells to undergo apoptosis by HIV-infected monocytes. A two-step mechanism involving the gp120 molecule. J. Clin. Investig. 99**:**257-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enders, J. F. 1954. Cytopathology of virus infections. Annu. Rev. Microbiol. 8**:**473-502. [DOI] [PubMed] [Google Scholar]
- 23.Estaquier, J., M. Tanaka, T. Suda, S. Nagata, P. Golstein, and J. C. Ameisen. 1996. Fas-mediated apoptosis of CD4+ and CD8+ T cells from human immunodeficiency virus-infected persons: differential in vitro preventive effect of cytokines and protease antagonists. Blood 87**:**4959-4966. [PubMed] [Google Scholar]
- 24.Fauci, A. S., and R. C. Desrosiers. 1997. Pathogenesis of HIV and SIV, p. 587-635. In J. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, New York, N.Y. [PubMed]
- 25.Finkel, T. H., G. Tudor-Williams, N. K. Banda, M. F. Cotton, T. Curiel, C. Monks, T. W. Baba, R. M. Ruprecht, and A. Kupfer. 1995. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat. Med. 1**:**129-134. [DOI] [PubMed] [Google Scholar]
- 26.Fisher, A. G., L. Ratner, H. Mitsuya, L. M. Marselle, M. E. Harper, S. Broder, R. C. Gallo, and F. Wong-Staal. 1986. Infectious mutants of HTLV-III with changes in the 3′ region and markedly reduced cytopathic effects. Science 233**:**655-659. [DOI] [PubMed] [Google Scholar]
- 27.Gandhi, R. T., B. K. Chen, S. E. Straus, J. K. Dale, M. J. Lenardo, and D. Baltimore. 1998. HIV-1 directly kills CD4+ T cells by a Fas-independent mechanism. J. Exp. Med. 187**:**1113-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibellini, D., A. Caputo, C. Celeghini, A. Bassini, M. La Placa, S. Capitani, and G. Zauli. 1995. Tat-expressing Jurkat cells show an increased resistance to different apoptotic stimuli, including acute human immunodeficiency virus-type 1 (HIV-1) infection. Br. J. Haematol. 89**:**24-33. [DOI] [PubMed] [Google Scholar]
- 29.Gougeon, M. L., S. Garcia, J. Heeney, R. Tschopp, H. Lecoeur, D. Guetard, V. Rame, C. Dauguet, and L. Montagnier. 1993. Programmed cell death in AIDS-related HIV and SIV infections. AIDS Res. Hum. Retrovir. 9**:**553-563. [DOI] [PubMed] [Google Scholar]
- 30.Gougeon, M. L., and L. Montagnier. 1993. Apoptosis in AIDS. Science 260**:**1269-1270. (Erratum, **260:**1709.) [DOI] [PubMed] [Google Scholar]
- 31.Groux, H., G. Torpier, D. Monte, Y. Mouton, A. Capron, and J. C. Ameisen. 1992. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J. Exp. Med. 175**:**331-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harper, M. E., L. M. Marselle, R. C. Gallo, and F. Wong-Staal. 1986. Detection of lymphocytes expressing human T-lymphotropic virus type III in lymph nodes and peripheral blood from infected individuals by in situ hybridization. Proc. Natl. Acad. Sci. USA 83**:**772-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He, J., S. Choe, R. Walker, P. Di Marzio, D. O. Morgan, and N. R. Landau. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 69**:**6705-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herbein, G., C. Van Lint, J. L. Lovett, and E. Verdin. 1998. Distinct mechanisms trigger apoptosis in human immunodeficiency virus type 1-infected and in uninfected bystander T lymphocytes. J. Virol. 72**:**660-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho, D. D., A. U. Neumann, A. S. Perelson, W. Chen, J. M. Leonard, and M. Markowitz. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373**:**123-126. [DOI] [PubMed] [Google Scholar]
- 36.Honda, O., M. Kuroda, I. Joja, J. Asaumi, Y. Takeda, S. Akaki, I. Togami, S. Kanazawa, S. Kawasaki, and Y. Hiraki. 2000. Assessment of secondary necrosis of jurkat cells using a new microscopic system and double staining method with Annexin V and propidium iodide. Int. J. Oncol. 16**:**283-288. [DOI] [PubMed] [Google Scholar]
- 37.Hrimech, M., X. J. Yao, F. Bachand, N. Rougeau, and E. A. Cohen. 1999. Human immunodeficiency virus type 1 (HIV-1) Vpr functions as an immediate-early protein during HIV-1 infection. J. Virol. 73**:**4101-4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacotot, E., C. Callebaut, J. Blanco, Y. Riviere, B. Krust, and A. G. Hovanessian. 1996. HIV envelope glycoprotein-induced cell killing by apoptosis is enhanced with increased expression of CD26 in CD4+ T cells. Virology 223**:**318-330. [DOI] [PubMed] [Google Scholar]
- 39.Jamieson, B. D., G. M. Aldrovandi, V. Planelles, J. B. Jowett, L. Gao, L. M. Bloch, I. S. Chen, and J. A. Zack. 1994. Requirement of human immunodeficiency virus type 1 nef for in vivo replication and pathogenicity. J. Virol. 68**:**3478-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kameoka, M., T. Kimura, Y. H. Zheng, S. Suzuki, K. Fujinaga, R. B. Luftig, and K. Ikuta. 1997. Protease-defective, gp120-containing human immunodeficiency virus type 1 particles induce apoptosis more efficiently than does wild-type virus or recombinant gp120 protein in healthy donor-derived peripheral blood T cells. J. Clin. Microbiol. 35**:**41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerr, J. F., A. H. Wyllie, and A. R. Currie. 1972. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26**:**239-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koester, S. K., P. Roth, W. R. Mikulka, S. F. Schlossman, C. Zhang, and W. E. Bolton. 1997. Monitoring early cellular responses in apoptosis is aided by the mitochondrial membrane protein-specific monoclonal antibody APO2.7. Cytometry 29**:**306-312. [PubMed] [Google Scholar]
- 43.Kolesnitchenko, V., L. King, A. Riva, Y. Tani, S. J. Korsmeyer, and D. I. Cohen. 1997. A major human immunodeficiency virus type 1-initiated killing pathway distinct from apoptosis. J. Virol. 71**:**9753-9763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolesnitchenko, V., L. M. Wahl, H. Tian, I. Sunila, Y. Tani, D. P. Hartmann, J. Cossman, M. Raffeld, J. Orenstein, L. E. Samelson, et al. 1995. Human immunodeficiency virus 1 envelope-initiated G2-phase programmed cell death. Proc. Natl. Acad. Sci. USA 92**:**11889-11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kowalski, M., L. Bergeron, T. Dorfman, W. Haseltine, and J. Sodroski. 1991. Attenuation of human immunodeficiency virus type 1 cytopathic effect by a mutation affecting the transmembrane envelope glycoprotein. J. Virol. 65**:**281-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laurent-Crawford, A. G., B. Krust, S. Muller, Y. Riviere, M. A. Rey-Cuille, J. M. Bechet, L. Montagnier, and A. G. Hovanessian. 1991. The cytopathic effect of HIV is associated with apoptosis. Virology 185**:**829-839. [DOI] [PubMed] [Google Scholar]
- 47.Lazdins, J. K., M. Grell, M. R. Walker, K. Woods-Cook, P. Scheurich, and K. Pfizenmaier. 1997. Membrane tumor necrosis factor (TNF) induced cooperative signaling of TNFR60 and TNFR80 favors induction of cell death rather than virus production in HIV-infected T cells. J. Exp. Med. 185**:**81-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leist, M., and M. Jaattela. 2001. Four deaths and a funeral: from caspases to alternative mechanisms. Nat. Rev. Mol. Cell. Biol. 2**:**589-598. [DOI] [PubMed] [Google Scholar]
- 49.Li, C. J., D. J. Friedman, C. Wang, V. Metelev, and A. B. Pardee. 1995. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science 268**:**429-431. [DOI] [PubMed] [Google Scholar]
- 50.Lu, W., R. Salerno-Goncalves, J. Yuan, D. Sylvie, D. S. Han, and J. M. Andrieu. 1995. Glucocorticoids rescue CD4+ T lymphocytes from activation-induced apoptosis triggered by HIV-1: implications for pathogenesis and therapy. AIDS 9**:**35-42. [DOI] [PubMed] [Google Scholar]
- 51.Lu, Y. Y., Y. Koga, K. Tanaka, M. Sasaki, G. Kimura, and K. Nomoto. 1994. Apoptosis induced in CD4+ cells expressing gp160 of human immunodeficiency virus type 1. J. Virol. 68**:**390-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maldarelli, F., H. Sato, E. Berthold, J. Orenstein, and M. A. Martin. 1995. Rapid induction of apoptosis by cell-to-cell transmission of human immunodeficiency virus type 1. J. Virol. 69**:**6457-6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manning, W. C., J. E. Murphy, D. J. Jolly, S. J. Mento, and R. O. Ralston. 1998. Use of a recombinant murine cytomegalovirus expressing vesicular stomatitis virus G protein to pseudotype retroviral vectors. J. Virol. Methods 73**:**31-39. [DOI] [PubMed] [Google Scholar]
- 54.McCloskey, T. W., M. Ott, E. Tribble, S. A. Khan, S. Teichberg, M. O. Paul, S. Pahwa, E. Verdin, and N. Chirmule. 1997. Dual role of HIV Tat in regulation of apoptosis in T cells. J. Immunol. 158**:**1014-1019. [PubMed] [Google Scholar]
- 55.Meyaard, L., and F. Miedema. 1997. Immune dysregulation and CD4+ T cell loss in HIV-1 infection. Springer Semin. Immunopathol. 18**:**285-303. [DOI] [PubMed] [Google Scholar]
- 56.Mitra, D., M. Steiner, D. H. Lynch, L. Staiano-Coico, and J. Laurence. 1996. HIV-1 upregulates Fas ligand expression in CD4+ T cells in vitro and in vivo: association with Fas-mediated apoptosis and modulation by aurintricarboxylic acid. Immunology 87**:**581-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mochizuki, H., J. P. Schwartz, K. Tanaka, R. O. Brady, and J. Reiser. 1998. High-titer human immunodeficiency virus type 1-based vector systems for gene delivery into nondividing cells. J. Virol. 72**:**8873-8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muro-Cacho, C. A., G. Pantaleo, and A. S. Fauci. 1995. Analysis of apoptosis in lymph nodes of HIV-infected persons. Intensity of apoptosis correlates with the general state of activation of the lymphoid tissue and not with stage of disease or viral burden. J. Immunol. 154**:**5555-5566. [PubMed] [Google Scholar]
- 59.Nicholson, D. W., A. Ali, N. A. Thornberry, J. P. Vaillancourt, C. K. Ding, M. Gallant, Y. Gareau, P. R. Griffin, M. Labelle, Y. A. Lazebnik, et al. 1995. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376**:**37-43. [DOI] [PubMed] [Google Scholar]
- 60.Nicholson, D. W., and N. A. Thornberry. 1997. Caspases: killer proteases. Trends Biochem. Sci. 22**:**299-306. [DOI] [PubMed] [Google Scholar]
- 61.Ohagen, A., S. Ghosh, J. He, K. Huang, Y. Chen, M. Yuan, R. Osathanondh, S. Gartner, B. Shi, G. Shaw, and D. Gabuzda. 1999. Apoptosis induced by infection of primary brain cultures with diverse human immunodeficiency virus type 1 isolates: evidence for a role of the envelope. J. Virol. 73**:**897-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ohnimus, H., M. Heinkelein, and C. Jassoy. 1997. Apoptotic cell death upon contact of CD4+ T lymphocytes with HIV glycoprotein-expressing cells is mediated by caspases but bypasses CD95 (Fas/Apo-1) and TNF receptor 1. J. Immunol. 159**:**5246-5252. [PubMed] [Google Scholar]
- 63.Okada, H., R. Takei, and M. Tashiro. 1998. Inhibition of HIV-1 Nef-induced apoptosis of uninfected human blood cells by serine/threonine protein kinase inhibitors, fasudil hydrochloride and M3. FEBS Lett. 422**:**363-367. [DOI] [PubMed] [Google Scholar]
- 64.Pan, Z., W. Radding, T. Zhou, E. Hunter, J. Mountz, and J. M. McDonald. 1996. Role of calmodulin in HIV-potentiated Fas-mediated apoptosis. Am. J. Pathol. 149**:**903-910. [PMC free article] [PubMed] [Google Scholar]
- 65.Park, I. W., E. Kondo, L. Bergeron, J. Park, and J. Sodroski. 1996. Effects of human immunodeficiency virus type 1 infection on programmed cell death in the presence or absence of Bcl-2. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 12**:**321-328. [DOI] [PubMed] [Google Scholar]
- 66.Patki, A. H., and M. M. Lederman. 1996. HIV-1 Tat protein and its inhibitor Ro 24-7429 inhibit lymphocyte proliferation and induce apoptosis in peripheral blood mononuclear cells from healthy donors. Cell. Immunol. 169**:**40-46. [DOI] [PubMed] [Google Scholar]
- 67.Plymale, D. R., D. S. Tang, A. M. Comardelle, C. D. Fermin, D. E. Lewis, and R. F. Garry. 1999. Both necrosis and apoptosis contribute to HIV-1-induced killing of CD4 cells. AIDS 13**:**1827-1839. [DOI] [PubMed] [Google Scholar]
- 68.Popovic, M., M. G. Sarngadharan, E. Read, and R. C. Gallo. 1984. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science 224**:**497-500. [DOI] [PubMed] [Google Scholar]
- 69.Rocancourt, D., C. Bonnerot, H. Jouin, M. Emerman, and J. F. Nicolas. 1990. Activation of a beta-galactosidase recombinant provirus: application to titration of human immunodeficiency virus (HIV) and HIV-infected cells. J. Virol. 64**:**2660-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sastry, K. J., M. C. Marin, P. N. Nehete, K. McConnell, A. K. el-Naggar, and T. J. McDonnell. 1996. Expression of human immunodeficiency virus type I tat results in downregulation of bcl-2 and induction of apoptosis in hematopoietic cells. Oncogene 13**:**487-493. [PubMed] [Google Scholar]
- 71.Savarino, A., C. Martini, G. C. Orofino, C. Cantamessa, L. Castelli, P. G. Pich, A. Sinicco, and A. Pugliese. 1997. Apoptotic DNA fragmentation, and its in vitro prevention by nicotinamide, in lymphocytes from HIV-1-seropositive patients and in HIV-1-infected MT-4 cells. Cell Biochem. Funct. 15**:**171-179. [DOI] [PubMed] [Google Scholar]
- 72.Shearer, G. M. 1998. HIV-induced immunopathogenesis. Immunity 9**:**587-593. [DOI] [PubMed] [Google Scholar]
- 73.Siliciano, R. F. 1996. The role of CD4 in HIV envelope-mediated pathogenesis. Curr. Top. Microbiol. Immunol. 205**:**159-179. [DOI] [PubMed] [Google Scholar]
- 74.Silvestris, F., S. Nagata, P. Cafforio, N. Silvestris, and F. Dammacco. 1996. Cross-linking of Fas by antibodies to a peculiar domain of gp120 V3 loop can enhance T cell apoptosis in HIV-1-infected patients. J. Exp. Med. 184**:**2287-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sloand, E. M., N. S. Young, P. Kumar, F. F. Weichold, T. Sato, and J. P. Maciejewski. 1997. Role of Fas ligand and receptor in the mechanism of T-cell depletion in acquired immunodeficiency syndrome: effect on CD4+ lymphocyte depletion and human immunodeficiency virus replication. Blood 89**:**1357-1363. [PubMed] [Google Scholar]
- 76.Swanstrom, R., and H. E. Wills. 1997. Synthesis, assembly, and processing of viral proteins, p. 263-334. In J. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [PubMed]
- 77.Temin, H. M. 1988. Mechanisms of cell killing/cytopathic effects by nonhuman retroviruses. Rev. Infect. Dis. 10**:**399-405. [DOI] [PubMed] [Google Scholar]
- 78.Terai, C., R. S. Kornbluth, C. D. Pauza, D. D. Richman, and D. A. Carson. 1991. Apoptosis as a mechanism of cell death in cultured T lymphoblasts acutely infected with HIV-1. J. Clin. Investig. 87**:**1710-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thompson, C. B. 1995. Apoptosis in the pathogenesis and treatment of disease. Science 267**:**1456-1462. [DOI] [PubMed] [Google Scholar]
- 80.Thompson, E. B. 1998. Special topic: apoptosis. Annu. Rev. Physiol. 60**:**525-532. [DOI] [PubMed] [Google Scholar]
- 81.Tian, H., R. Lempicki, L. King, E. Donoghue, L. E. Samelson, and D. I. Cohen. 1996. HIV envelope-directed signaling aberrancies and cell death of CD4+ T cells in the absence of TCR costimulation. Int. Immunol. 8**:**65-74. [DOI] [PubMed] [Google Scholar]
- 82.Tuosto, L., M. S. Montani, S. Lorenzetti, E. Cundari, S. Moretti, G. Lombardi, and E. Piccolella. 1995. Differential susceptibility to monomeric HIV gp120-mediated apoptosis in antigen-activated CD4+ T cell populations. Eur. J. Immunol. 25**:**2907-2916. [DOI] [PubMed] [Google Scholar]
- 83.Vercammen, D., R. Beyaert, G. Denecker, V. Goossens, G. Van Loo, W. Declercq, J. Grooten, W. Fiers, and P. Vandenabeele. 1998. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J. Exp. Med. 187**:**1477-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wagner, R. R. 1980. Cytopathic effects of viruses: a general survey. Comp. Virol. 19**:**1-63. [Google Scholar]
- 85.Wei, X., S. K. Ghosh, M. E. Taylor, V. A. Johnson, E. A. Emini, P. Deutsch, J. D. Lifson, S. Bonhoeffer, M. A. Nowak, B. H. Hahn, et al. 1995. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373**:**117-122. [DOI] [PubMed] [Google Scholar]
- 86.Weller, S. K., A. E. Joy, and H. M. Temin. 1980. Correlation between cell killing and massive second-round superinfection by members of some subgroups of avian leukosis virus. J. Virol. 33**:**494-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Westendorp, M. O., R. Frank, C. Ochsenbauer, K. Stricker, J. Dhein, H. Walczak, K. M. Debatin, and P. H. Krammer. 1995. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 375**:**497-500. [DOI] [PubMed] [Google Scholar]
- 88.Wyllie, A. H., J. F. Kerr, and A. R. Currie. 1980. Cell death: the significance of apoptosis. Int. Rev. Cytol. 68**:**251-306. [DOI] [PubMed] [Google Scholar]
- 89.Xu, X. N., B. Laffert, G. R. Screaton, M. Kraft, D. Wolf, W. Kolanus, J. Mongkolsapay, A. J. McMichael, and A. S. Baur. 1999. Induction of Fas ligand expression by HIV involves the interaction of Nef with the T cell receptor zeta chain. J. Exp. Med. 189**:**1489-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang, Y., Z. H. Liu, C. F. Ware, and J. D. Ashwell. 1997. A cysteine protease inhibitor prevents activation-induced T-cell apoptosis and death of peripheral blood cells from human immunodeficiency virus-infected individuals by inhibiting upregulation of Fas ligand. Blood 89**:**550-557. [PubMed] [Google Scholar]
- 91.Zauli, G., D. Gibellini, A. Caputo, A. Bassini, M. Negrini, M. Monne, M. Mazzoni, and S. Capitani. 1995. The human immunodeficiency virus type-1 Tat protein upregulates Bcl-2 gene expression in Jurkat T-cell lines and primary peripheral blood mononuclear cells. Blood 86**:**3823-3834. [PubMed] [Google Scholar]
- 92.Zhang, Y. J., B. Fadeel, V. Hodara, and E. M. Fenyo. 1997. Induction of apoptosis by primary HIV-1 isolates correlates with productive infection in peripheral blood mononuclear cells. AIDS 11**:**1219-1225. [DOI] [PubMed] [Google Scholar]