Convergence of Wnt, β-Catenin, and Cadherin Pathways (original) (raw)
. Author manuscript; available in PMC: 2012 Jun 12.
Published in final edited form as: Science. 2004 Mar 5;303(5663):1483–1487. doi: 10.1126/science.1094291
Abstract
The specification and proper arrangements of new cell types during tissue differentiation require the coordinated regulation of gene expression and precise interactions between neighboring cells. Of the many growth factors involved in these events, Wnts are particularly interesting regulators, because a key component of their signaling pathway, β-catenin, also functions as a component of the cadherin complex, which controls cell-cell adhesion and influences cell migration. Here, we assemble evidence of possible interrelations between Wnt and other growth factor signaling, β-catenin functions, and cadherin-mediated adhesion.
During embryogenesis, cells often acquire new identities as they migrate to new locations (1). Many of these morphogenetic changes are induced by extracellular ligands and their receptors (1–4). An important problem is to identify the signaling pathways that coordinate changes in gene expression with dynamic changes in cell adhesion and migration. Deregulation of these pathways is likely to lead to alterations in cell fate, adhesion, and migration, hallmarks of diseases such as cancer.
Although several growth factors are known to affect both gene expression and cell migration (3), recent focus has been on the Wnt signaling pathway. Wnts are powerful regulators of cell proliferation and differentiation, and their signaling pathway involves proteins that directly participate in both gene transcription and cell adhesion. The central player is β-catenin, which is a transcription cofactor with T cell factor/lymphoid enhancer factor TCF/LEF in the Wnt pathway (2) and a structural adaptor protein linking cadherins to the actin cytoskeleton in cell-cell adhesion (5). This review explores intriguing connections between Wnt and other growth factor signals, β-catenin distribution, and cadherin-mediated cell adhesion (Fig. 1, inset).
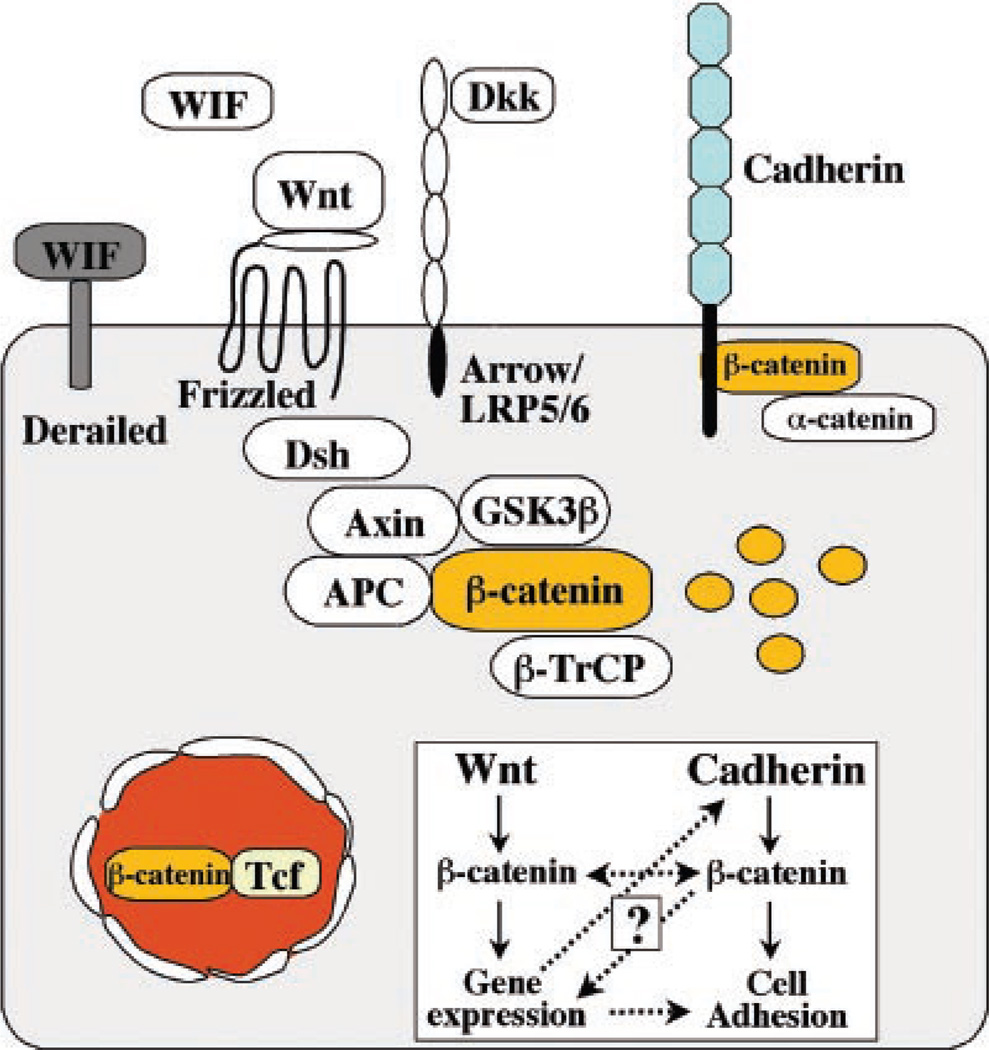
Fig. 1.
The central role of β-catenin in Wnt signaling and the cadherin complex. β-Catenin exists in a cadherin-bound form that regulates adhesion; in a complex with axin, APC, and GSK-3β, where it is phosphorylated and targeted for degradation by β-TrCP; or in the nucleus with TCF/LEF transcription factors. Wnt signaling, proceeding through Frizzled and Arrow–LRP-5/6, activates Dishevelled (Dsh), which results in uncoupling β-catenin from the degradation pathway and its entry into the nucleus, where it interacts with TCF/LEF to control transcription. Wnt protein can also interact with the Derailed receptor to control axon path-finding. The Wnt pathway is also subject to extensive regulation and feedback control by extracellular factors that bind Wnt [Wnt inhibitory factor (WIF) and Frizzled-related protein (FRP)] or the coreceptor LRP (Dickkopf). The insert displays possible levels of interactions between Wnt signaling and cadherin-mediated adhesion (dotted lines) and the central role of β-catenin in both processes that are the focus of the review.
The Wnt Signaling Pathway and Control of β-Catenin Levels
Wnts are secreted lipid-modified signaling proteins (6) that influence multiple processes in animal development. Nineteen Wnt genes exist in mammalian genomes, and the diversity of their functions is exemplified by mutations that lead to developmental abnormalities ranging from stem cell loss to kidney and reproductive tract defects (2). Signaling is initiated by Wnt ligand binding to two receptor molecules, Frizzled proteins and lipoprotein receptor–related proteins 5 and 6 (LRP-5/6) (Fig. 1).
Conventional Wnt signaling causes β-catenin accumulation in a complex with the transcription factor TCF/LEF that regulates target gene expression (Fig. 1). In the absence of Wnt signaling, the level of β-catenin is kept low through degradation of (cytoplasmic) β-catenin that is in excess of binding sites, such as cadherins at the plasma membrane (see below). β-Catenin is targeted for ubiquitination and degradation in the 26_S_ proteosome by paired phosphorylation through the serine/threonine kinases casein kinase I (CKI) and glycogen synthase-3 β(GSK-3β) (7) bound to a scaffolding complex of axin and adenomatous polyposis coli (APC) protein (2, 8). Activation of Wnt signaling leads to inhibition of GSK-3β activity, resulting in accumulation of cytoplasmic (signaling) β-catenin, which becomes available to bind the TCF/LEF family of transcription factors and to induce target gene expression (2). Thus, the key factors in β-catenin signaling are its stabilization and accumulation in the cytoplasm.
Control of Cadherin Function in Cell Adhesion and Sequestering β-Catenin
In addition to its function in the Wnt signaling pathway, β-catenin also binds tightly to the cytoplasmic domain of type I cadherins and plays an essential role in the structural organization and function of cadherins by linking cadherins through α-catenin to the actin cytoskeleton (Fig. 2) (5, 9). Another catenin, p120, binds to the membrane proximal domain of cadherin and regulates the structural integrity and function of the cadherin complex (10). Can the cadherin-bound pool of β-catenin be released and made available for signaling? To answer this question, it is important to understand how the dynamic interaction of β-catenin with cadherin is regulated (Fig. 2).
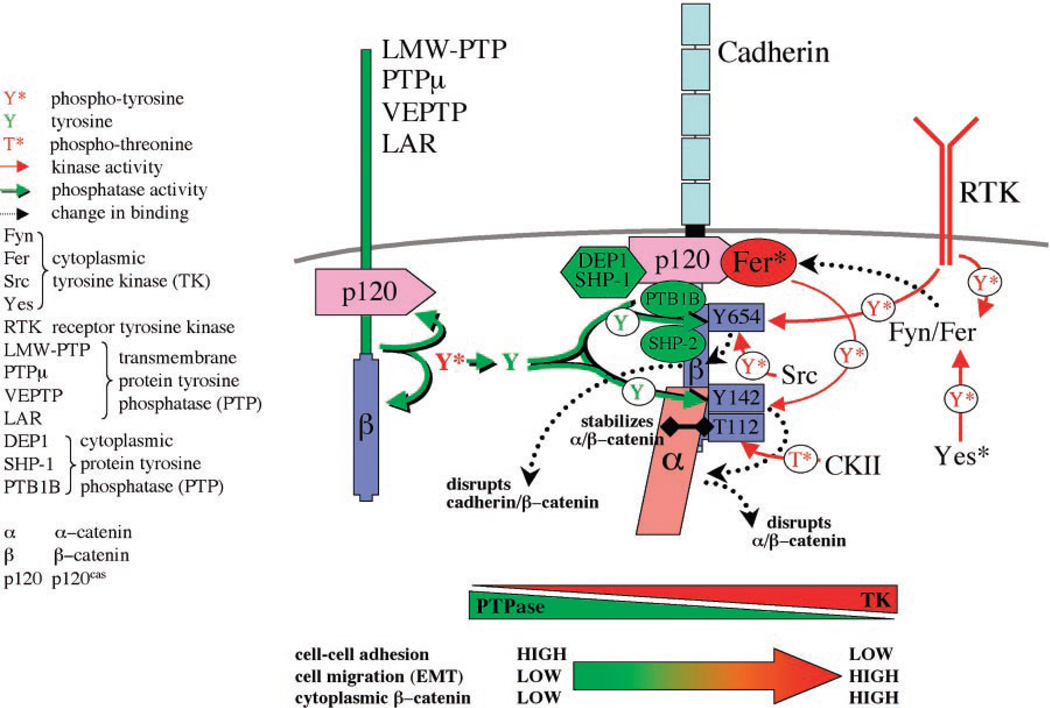
Fig. 2.
Structural and functional regulation of the cadherin-catenin complex by the balance of tyrosine kinase and phosphatase activities. Cadherin binds p120 and β-catenin, which in turn binds α-catenin. The integrity of this complex is negatively regulated by phosphorylation of β-catenin by receptor tyrosine kinases (RTKs) and cytoplasmic tyrosine kinases (Fer, Fyn, Yes, and Src), which phosphorylate (red arrows) specific tyrosine residues in β-catenin (Y654, Y142), which leads to dissociation of the cadherin-catenin complex. Integrity of the cadherin-catenin complex is positively regulated by β-catenin phosphorylation by casein kinase II, and dephosphorylation by protein tyrosine phosphatases that bind p120 and β-catenin (green arrows). Changes in the phosphorylation state of β-catenin (bottom) affect cell-cell adhesion, cell migration, and the level of signaling β-catenin.
The structural and functional integrity of the cadherin-catenin complex is regulated by phosphorylation (11). Serine/threonine phosphorylation of β-catenin (12) or epithelial cadherin (E-cadherin) (13) results in increased stabilization of the cadherin-catenin complex. However, tyrosine phosphorylation of β-catenin by the cytoplasmic kinase Fer disrupts binding of β-catenin to α-catenin (14), whereas phosphorylation by Src or the epidermal growth factor (EGF) receptor (15) disrupts binding of β-catenin to cadherin. Phosphorylation of p120 by Src (15) or Fer (16) results in loss of cadherin complexes from the cell surface, perhaps as a consequence of simultaneous phosphorylation of β-catenin or because p120 is a binding site for several protein tyrosine phosphatases (PTPases) that antagonize the effects of these tyrosine kinases. In general, activation of tyrosine kinases results in a loss of cadherin-mediated cell-cell adhesion and an increase in the level of cytoplasmic β-catenin (14, 15), either by direct release of β-catenin into the cytoplasm or by activating cadherin endocytosis (17). In contrast, activation of PTPases stabilizes the cadherin-catenin complex and results in increased cadherin-mediated cell-cell adhesion (18–20). Although many of these studies were conducted with tissue culture cells, the role of phosphorylation in regulating the organization and function of the cadherin-catenin complex is supported by studies with endothelial cells (19, 21) and preimplantation embryos (22).
Several studies with tissue culture cells show that activation of tyrosine kinases can increase β-catenin signaling in the nucleus (Fig. 3). For example, activation of oncogenic RON receptor tyrosine kinase (RTK) or the receptor for hepatocyte growth factor, cMET, results in tyrosine phosphorylation of β-catenin, accumulation of β-catenin, and increased TCF-mediated gene transcription (23). Conversely, inactivation of the EGF receptor ErbB2 results in increased binding of β-catenin to cadherin and a corresponding decrease in TCF/β-catenin–mediated gene transcription (24). However, as noted earlier, activation of signaling β-catenin requires its stabilization and accumulation in the cytoplasm. Indeed, activation of insulin-like growth factor (IGF) type II receptor leads to transfer of β-catenin to the nucleus and TCF/LEF-mediated gene transcription (25), but in the case of type I receptor this occurs only when β-catenin is first stabilized (26). Although much remains to be learned about these pathways, particularly in the physiological context of cells in tissues, it is important to consider that phosphorylation-dependent release of β-catenin from the cadherin complex not only regulates the integrity and function of the adhesion complex, but may also be an alternative mechanism for activating β-catenin signaling.
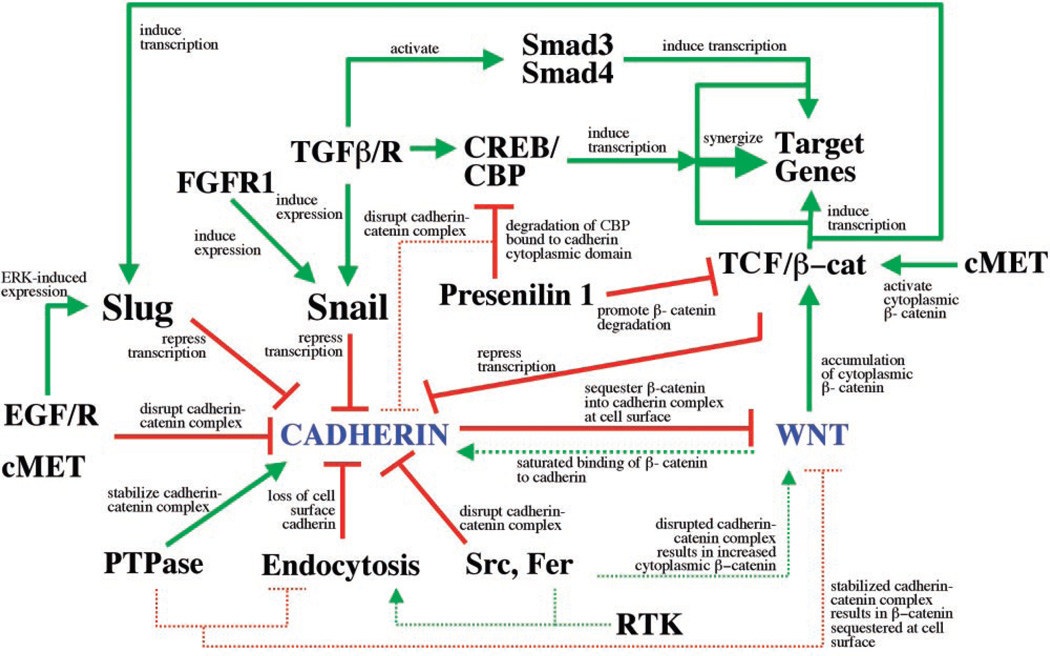
Fig. 3.
Intersection of pathways controlling Wnt/β-catenin signaling and cadherin-mediated adhesion. Connections between cadherin and Wnt/β-catenin signaling pathways are based on studies in tissue culture cells and in tissues, and some involve manipulations of protein levels and expression patterns (for details, see text). All possible intersections between these pathways and their outcomes are represented together as a map, although individual pathways are likely to occur only in specific physiological contexts. Pathways that activate are indicated by solid green, pathways that reduce activity are indicated in solid red, and indirect consequences of pathway activation or inactivation are indicated by dotted lines.
The intersection of pathways regulating the cadherin-catenin complex with β-catenin stability and signaling is further exemplified by newly uncovered functions of presenilin 1 (PS1), the major gene responsible for familial Alzheimer’s disease (27). Like the axin/APC scaffold complex, PS1 appears to regulate β-catenin stability by facilitating its paired phosphorylation by CKI and GSK-3β and subsequent degradation (28, 29). In cells and tissues from _PS1_−/− mice, β-catenin is stabilized and accumulates in the nucleus (30), which indicates that the axin/APC scaffold expressed in those cells does not have the capacity to target all available β-catenin for degradation. In addition to showing that PS1 can directly affect β-catenin levels, studies using cell extracts from _PS1_−/− and PS1+/+ mice showed that PS1 can also proteolytically cleave the cytoplasmic domain of cadherin, which results in a loss of cell-cell adhesion (31). The released cytoplasmic fragment of cadherin binds the CREB-binding protein (CBP) and targets it for degradation (32). CBP is a scaffold for activating transcriptional modulators of the cAMP response element– binding protein (CREB) basal transcription complex (33), and CBP degradation results in the suppression of CREB-mediated transcription (32). Although further studies are needed, these new activities of PS1 identify a Wnt-independent pathway that regulates and interconnects β-catenin functions in both the cadherin complex and signaling to the nucleus (Fig. 3).
Control of Cadherin Expression and β-Catenin Signaling by Wnt and Other Growth Factor Pathways
Early studies sought to examine whether the cadherin-bound and signaling pools of β-catenin were mutually exclusive (Fig. 3). Overexpression of cadherins in Xenopus and Drosophila embryos reduced the availability of β-catenin by sequestering it at the plasma membrane and thereby made it unavailable for signaling to the nucleus (34, 35). However, increased levels of β-catenin induced by Wnt signaling in tissue culture cells led to saturation of β-catenin binding to cadherin at the plasma membrane and an increase in cell-cell adhesion (36). These results point to a potential role of cadherin in sequestering signaling β-catenin at the plasma membrane, although an important caveat in those experiments is that cadherin and β-catenin levels were artificially manipulated. Nevertheless, it is interesting to consider this function further in light of recent results that have connected changes in E-cadherin gene transcription, loss of cell-cell adhesion, and Wnt/β-catenin signaling.
Zinc finger proteins of the Slug/Snail family are repressors of E-cadherin gene transcription (37–39), and their expression is activated by signaling from the fibroblast growth factor FGF-R type I (40), transforming growth factor–β (TGFβ1) (41), and ErbB1 and ErbB2 (39). Slug/Snail expression results in loss of cell-cell adhesion and increased cell migration (3), as well as accumulation of signaling β-catenin that may function independently of, or synergize with, Wnt signaling (40). Significantly, Wnt signaling is attenuated in _Fgfr_−/− mice, but signaling can be rescued by lowering E-cadherin levels (40). Wnt signaling also regulates E-cadherin expression, as studies in tissue culture cells and in tissues have shown that Slug may be a target gene of TCF/β-catenin complex (39) and that the TCF/β-catenin complex binds to and represses the E-cadherin promoter (42). Thus, repression of cadherin expression by Slug/Snail or TCF/β-catenin complex not only reduces cell-cell adhesion, but the concomitant accumulation of signaling β-catenin may lower the threshold for activating Wnt signaling and, thereafter, amplify and/or sustain Wnt signaling.
Activation of specific gene transcription by β-catenin is also coordinated by TGFβ signaling and its downstream effectors (Smads) in the nucleus (Fig. 3). Genes activated by Wnt signaling alone, or by both TGFβ and Wnt, are different (43, 44). Significantly, activities of Smads, TCF/LEF, and β-catenin appear to synergize to increase gene transcription such that any one of the proteins alone activates transcription at a base level, which increases to a maximum when all three proteins are involved (43, 45). Together, these results show that a variety of growth factor/receptor pathways intersect with the Wnt pathway by regulating the availability of signaling β-catenin, either by disrupting the cadherin-catenin complex or by repressing cadherin expression.
Alternative Roles of Wnt Signaling Components in Cell Adhesion and Migration
Components of the Wnt pathway are involved in morphogenetic processes that may not directly involve transcriptional end points but signaling toward the cytoskeleton and cell polarity in tissues. One pathway best understood in Drosophila is called planar polarity signaling, but it is also implicated in epithelial tissue polarity in vertebrates (46). Planar polarity is regulated by Frizzled, Dishevelled, and several other components including RhoA (Fig. 4A). Available evidence suggests that Frizzled is activated by the interaction between the nonclassical cadherin-like cell adhesion proteins, Fat and Dachsous (47). Together with Four Jointed, a membrane-anchored signaling molecule, these proteins are responsible for setting up asymmetry in cells (47). Among the known other players in planar polarity is another adhesion molecule, Flamingo (48), which has an unusual structure of a cadherin-like extracellular domain followed by a domain that resembles a seven-transmembrane signaling moiety (Fig. 4A). How these cell adhesion protein complexes control cell polarity is poorly understood (46), although this pathway does provide a further link between some components of the Wnt signaling pathway and cell-cell adhesion.
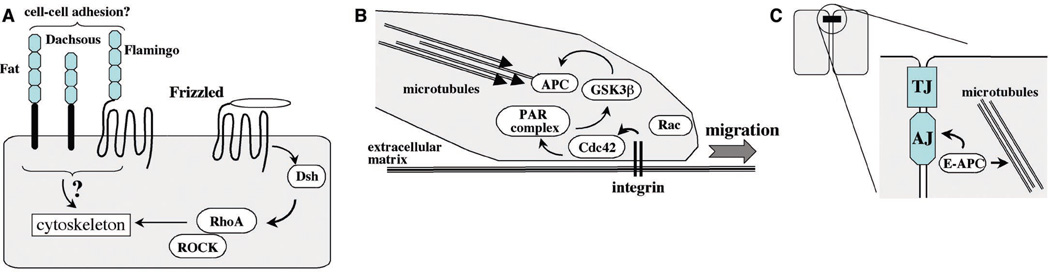
Fig. 4.
Additional roles of Wnt signaling components, adhesion proteins, and APC. (A) Planar polarity in Drosophila is regulated by Frizzled signaling through Dsh, the small guanosine triphosphatase (GTPase) RhoA, and its downstream effector Rho-kinase (ROCK). Although it is not known whether this process is Wnt-dependent, it is controlled by nonclassical cadherin-like adhesion molecules of the Fat-Dachsous family, as well as by Flamingo. All these molecules contain cadherin repeats, that, in the case of Flamingo, are linked to a seven-transmembrane domain. The number of cadherin repeats displayed here is arbitrary. There are numerous additional components in planar polarity not discussed here (46). (B) In addition to a role in targeting β-catenin for degradation, APC also interacts with the plus-end of microtubules at the plasma membrane of migrating cells. Recent studies indicate that APC and microtubules orient the direction of cell migration through a signaling cascade from integrins that bind extracellular matrix; the small GTPase Cdc42; the PAR complex, which contains the scaffolding proteins Par3/Par 6 and an atypical protein kinase C; and serine/threonine kinase GSK-3β (50–52). (C) APC also localizes with the cadherin-catenin complex at the adherens junction (AJ), a major cell-cell adhesion complex with the tight junction (TJ) at the boundary between the apical and lateral membranes of polarized epithelial cells; E-APC may linkmicrotubules to the plasma membrane and may regulate the organization and function of the AJ (45, 53).
Another component of the Wnt signaling pathway, APC, is involved in polarized cell migration and cell-cell adhesion (Fig. 4, B and C). Early studies showed that APC is localized to the tips of plasma membrane projections in migrating cells in association with bundles of microtubules (49). More recently, this APC distribution has been linked to the activation of a signaling complex by integrin-based adhesion that orients the cell for polarized migration (50–52) (Fig. 4B). In addition, an APC homolog (E-APC) has been localized to the adherens junction in association with cadherin and β-catenin; mutations that disrupt E-APC localization disrupt cell-cell adhesion (53) and increase the level of cytoplasmic β-catenin (54) (Fig. 4C). Thus, APC is a multifunctional protein that provides further links between cell-cell adhesion and β-catenin stability and is involved in processes that are not linked directly to Wnt signaling but that contribute to cellular morphogenesis.
Origins of Wnt Signaling and Cell Adhesion
The multiple nodes that link cell adhesion to Wnt signaling indicate that these two processes have coevolved. Although such speculations are often difficult to test, some support may be gained by examining species with a more primitive body plan. In the sea anemone Nematostella vectensis, translocation of β-catenin to the nucleus is required to specify entoderm (55). Although the role of cadherins was not investigated in this system, overexpression of cadherin reduced nuclear β-catenin and inhibited gastrulation (55).
The diploblast Hydra has a well-developed cell adhesion system including Ca2+-dependent adhesion and catenins similar to vertebrate counterparts, and most components of the Wnt pathway including β-catenin, TCF, and Frizzled have been identified in the Hydra genome (56). Cell adhesion and Wnt signaling appear to regulate formation of a body axis during asexual budding; aggregation of individual cells is required to initiate TCF and Wnt expression, which leads to the polarized outgrowth and formation of a new body axis (56). Therefore, in these primitive organisms, as in mammals, there is a mechanistic link between cell-cell adhesion and Wnt signaling.
In considering the possibility of a common origin of Wnt and adhesion signaling, it should be emphasized that in at least one organism, Caenorhabditis elegans, these pathways are genetically separate. The C. elegans genome contains three β-catenin–related genes: HMP-2, which is dedicated to adhesion only, and BAR-1 and WRM-1, which act in Wnt signaling (57).
Wnt Signaling and Cell Adhesion Intersect in Stem Cell Organization and Cancer
Recent studies have shown that both Wnt signaling and cadherin-mediated cell-cell adhesion are important in the organization and maintenance of stem cells. In the Drosophila ovary, somatic and germline stem cells contact specialized cap cells of the germarium during late larval development (58). Wnt is expressed in these cap cells, close to the stem cells, and loss of Wnt signaling results in a concomitant loss of somatic stem cells (59). The cadherin/β-catenin complex, on the other hand, accumulates between germline stem cells (GSCs) and cap cells, and loss of this complex results in GSC loss (60).
One role of cadherin-mediated adhesion may be in regulating the orientation of cell division of GSCs. In the Drosophila testis, GSC mitosis gives rise to a stem cell and a gonialblast, and proper orientation of the division plane is required to maintain the GSC population. The orientation of this division is determined by adhesion between GSCs and the “hub,” a cluster of somatic cells. Adhesion between hub cells and GSCs is maintained by the cadherin–β-catenin complex, and by APC, which binds microtubules and functions to orient the mitotic spindle (61).
Both Wnt signaling and cadherin-mediated cell adhesion are also important in maintaining mammalian hematopoietic stem cells (HSCs). Wnt signaling and nuclear functions of β-catenin are required for HSCs to proliferate and to limit their differentiation potential (62). Maintenance of the HSC niche in the bone marrow appears to be dependent in part on HSC attachment to spindle-shaped osteoblast cells through neural cadherin–mediated cell-cell adhesion (63). Thus, Wnt signaling and correct orientation of cells and the mitotic spindle through cadherin-mediated cell-cell adhesion together contribute to regulate the stem cell niche.
Alterations in cell fate, adhesion, and migration are characteristics of cancer in which cells ignore normal regulatory cues from their environment. Unchecked Wnt signaling (8) and/or the loss of cell-cell adhesion (3, 64, 65) are involved in cancer induction and progression. A key event in cancer is the loss of control over β-catenin levels, which can be the consequence of loss-of-function mutations in APC, originally discovered because they predispose to colorectal cancer (8). In addition, activating mutations in β-catenin, of the kind that makes the molecule refractive to down-regulation by the APC–axin–GSK-3β destruction complex, are characteristic of some cancers (8). Loss of cadherin expression can also promote tumorigenesis (3, 64–66), although a link to activation of β-catenin signaling after the loss of cadherin remains unclear.
Conclusions
Progress in understanding Wnt and β-catenin signaling, as well as cadherin-mediated cell adhesion, has generally followed separate lines of investigation. However, it is clear that there are many connections between these pathways (Figs. 1 and 3), although some caution may be needed in interpreting studies in tissue culture cells and in cases where protein levels were artificially manipulated. The critical component in these pathways is β-catenin, and the key events are the regulation of β-catenin stability and availability. Wnt signaling acts as a positive regulator by inhibiting β-catenin degradation, which stabilizes β-catenin, and causes its accumulation. The scaffolding complexes of APCaxin, and possibly PS1, act as negative regulators as they target β-catenin for degradation, which reduces the overall level of cytoplasmic (signaling) β-catenin. Cadherin may also act as a negative regulator of signaling β-catenin as it binds β-catenin at the cell surface and thereby sequesters it from the nucleus. Negative regulation of signaling β-catenin by cadherin may be antagonized by growth factor receptors and tyrosine kinases that either down-regulate E-cadherin transcription through activation of the repressors Slug/Snail or disrupt the cadherin-catenin complex at the cell surface, either of which could lead to a coordinate decrease in cell-cell adhesion and increase in β-catenin signaling (Figs. 2 and 3). Although further studies are required to examine these connections in more complex physiological contexts, a picture is emerging in which components of the Wnt and cadherin pathways are not only linked through the activities of β-catenin, but may also be interconnected by regulatory loops that allow close coordination of gene expression and cell adhesion.
References
- 1.Affolter M, et al. Dev. Cell. 2003;4:11. doi: 10.1016/s1534-5807(02)00410-0. [DOI] [PubMed] [Google Scholar]
- 2.Cadigan K, Nusse R. Genes Dev. 1997;11:3286. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 3.Thiery JP. Nature Rev. Cancer. 2002;2:442. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 4.Massague J, Blain SW, Lo RS. Cell. 2000;103:295. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 5.Jamora C, Fuchs E. Nature Cell Biol. 2002;4:E101. doi: 10.1038/ncb0402-e101. [DOI] [PubMed] [Google Scholar]
- 6.Willert K, et al. Nature. 2003;423:448. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 7.Polakis P. Curr. Biol. 2002;12:R499. doi: 10.1016/s0960-9822(02)00969-7. [DOI] [PubMed] [Google Scholar]
- 8.Polakis P. Genes Dev. 2000;14:1837. [PubMed] [Google Scholar]
- 9.Gumbiner BM. J. Cell Biol. 2000;148:399. doi: 10.1083/jcb.148.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis MA, Ireton RC, Reynolds AB. J. Cell Biol. 2003;163:525. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lilien J, Balsamo J, Arregui C, Xu G. Dev. Dyn. 2002;224:18. doi: 10.1002/dvdy.10087. [DOI] [PubMed] [Google Scholar]
- 12.Bek S, Kemler R. J. Cell Sci. 2002;115:4743. doi: 10.1242/jcs.00154. [DOI] [PubMed] [Google Scholar]
- 13.Lickert H, Bauer A, Kemler R, Stappert J. J. Biol. Chem. 2000;275:5090. doi: 10.1074/jbc.275.7.5090. [DOI] [PubMed] [Google Scholar]
- 14.Piedra J, et al. Mol. Cell. Biol. 2003;23:2287. doi: 10.1128/MCB.23.7.2287-2297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M. J. Biol. Chem. 1999;274:36734. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- 16.Rosato R, Veltmaat JM, Groffen J, Heisterkamp N. Mol. Cell. Biol. 1998;18:5762. doi: 10.1128/mcb.18.10.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le TL, Yap AS, Stow JL. J. Cell Biol. 1999;146:219. [PMC free article] [PubMed] [Google Scholar]
- 18.Hellberg CB, Burden-Gulley SM, Pietz GE, Brady-Kalnay SM. J. Biol. Chem. 2002;277:11165. doi: 10.1074/jbc.M112157200. [DOI] [PubMed] [Google Scholar]
- 19.Nawroth R, et al. EMBO J. 2002;21:4885. doi: 10.1093/emboj/cdf497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balsamo J, Arregui C, Leung T, Lilien J. J. Cell Biol. 1998;143:523. doi: 10.1083/jcb.143.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grazia Lampugnani M, et al. J. Cell Biol. 2003;161:793. doi: 10.1083/jcb.200209019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohsugi M, Butz S, Kemler R. Dev. Dyn. 1999;216:168. doi: 10.1002/(SICI)1097-0177(199910)216:2<168::AID-DVDY7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 23.Danilkovitch-Miagkova A, et al. Mol. Cell. Biol. 2001;21:5857. doi: 10.1128/MCB.21.17.5857-5868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonvini P, et al. Cancer Res. 2001;61:1671. [PubMed] [Google Scholar]
- 25.Morali OG, et al. Oncogene. 2001;20:4942. doi: 10.1038/sj.onc.1204660. [DOI] [PubMed] [Google Scholar]
- 26.Playford MP, Bicknell D, Bodmer WF, Macaulay VM. Proc. Natl. Acad. Sci. U.S.A. 2000;97:12103. doi: 10.1073/pnas.210394297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haass C, De Strooper B. Science. 1999;286:916. doi: 10.1126/science.286.5441.916. [DOI] [PubMed] [Google Scholar]
- 28.Soriano S, et al. J. Cell Biol. 2001;152:785. doi: 10.1083/jcb.152.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang DE, et al. J. Neurosci. 1999;19:4229. doi: 10.1523/JNEUROSCI.19-11-04229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang DE, et al. Cell. 2002;110:751. doi: 10.1016/s0092-8674(02)00970-4. [DOI] [PubMed] [Google Scholar]
- 31.Marambaud P, et al. EMBO J. 2002;21:1948. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marambaud P, et al. Cell. 2003;114:635. doi: 10.1016/j.cell.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Goodman RH, Smolik S. Genes Dev. 2000;14:1553. [PubMed] [Google Scholar]
- 34.Heasman J, et al. Cell. 1994;79:791. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 35.Sanson B, White P, Vincent JP. Nature. 1996;383:627. doi: 10.1038/383627a0. [DOI] [PubMed] [Google Scholar]
- 36.Hinck L, Nelson WJ, Papkoff J. J. Cell Biol. 1994;124:729. doi: 10.1083/jcb.124.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cano A, et al. Nature Cell Biol. 2000;2:76. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 38.Batlle E, et al. Nature Cell Biol. 2000;2:84. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 39.Conacci-Sorrell M, et al. J. Cell Biol. 2003;163:847. doi: 10.1083/jcb.200308162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ciruna B, Rossant J. Dev. Cell. 2001;1:37. doi: 10.1016/s1534-5807(01)00017-x. [DOI] [PubMed] [Google Scholar]
- 41.Peinado H, Quintanilla M, Cano A. J. Biol. Chem. 2003;278:21113. doi: 10.1074/jbc.M211304200. [DOI] [PubMed] [Google Scholar]
- 42.Jamora C, DasGupta R, Kocieniewski P, Fuchs E. Nature. 2003;422:317. doi: 10.1038/nature01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishita M, et al. Nature. 2000;403:781. doi: 10.1038/35001602. [DOI] [PubMed] [Google Scholar]
- 44.Riese J, et al. Cell. 1997;88:777. doi: 10.1016/s0092-8674(00)81924-8. [DOI] [PubMed] [Google Scholar]
- 45.Labbe E, Letamendia A, Attisano L. Proc. Natl. Acad. Sci. U.S.A. 2000;97:8358. doi: 10.1073/pnas.150152697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veeman MT, Axelrod JD, Moon RT. Dev. Cell. 2003;5:367. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 47.Yang CH, Axelrod JD, Simon MA. Cell. 2002;108:675. doi: 10.1016/s0092-8674(02)00658-x. [DOI] [PubMed] [Google Scholar]
- 48.Usui T, et al. Cell. 1999;98:585. doi: 10.1016/s0092-8674(00)80046-x. [DOI] [PubMed] [Google Scholar]
- 49.Nathke IS, Adams CL, Polakis P, Sellin JH, Nelson WJ. J. Cell Biol. 1996;134:165. doi: 10.1083/jcb.134.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Etienne-Manneville S, Hall A. Cell. 2001;106:489. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 51.Etienne-Manneville S, Hall A. Nature. 2003;421:753. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- 52.Shi SH, Jan LY, Jan YN. Cell. 2003;112:63. doi: 10.1016/s0092-8674(02)01249-7. [DOI] [PubMed] [Google Scholar]
- 53.Hamada F, Bienz M. Nature Cell Biol. 2002;4:208. doi: 10.1038/ncb755. [DOI] [PubMed] [Google Scholar]
- 54.Yu X, Waltzer L, Bienz M. Nature Cell Biol. 1999;1:144. doi: 10.1038/11064. [DOI] [PubMed] [Google Scholar]
- 55.Wikramanayake AHH, et al. Nature. 2003;426:446. doi: 10.1038/nature02113. [DOI] [PubMed] [Google Scholar]
- 56.Hobmayer B, et al. Nature. 2000;407:186. doi: 10.1038/35025063. [DOI] [PubMed] [Google Scholar]
- 57.Korswagen HC, Herman MA, Clevers HC. Nature. 2000;406:527. doi: 10.1038/35020099. [DOI] [PubMed] [Google Scholar]
- 58.Gonzalez-Reyes A. J. Cell Sci. 2003;116:949. doi: 10.1242/jcs.00310. [DOI] [PubMed] [Google Scholar]
- 59.Song X, Xie T. Development. 2003;130:3259. doi: 10.1242/dev.00524. [DOI] [PubMed] [Google Scholar]
- 60.Song X, Zhu CH, Doan C, Xie T. Science. 2002;296:1855. doi: 10.1126/science.1069871. [DOI] [PubMed] [Google Scholar]
- 61.Yamashita YM, Jones DL, Fuller MT. Science. 2003;301:1547. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- 62.Reya T, et al. Nature. 2003;423:409. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 63.Zhang J, et al. Nature. 2003;425:836. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 64.Christofori G. EMBO J. 2003;22:2318. doi: 10.1093/emboj/cdg228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pagliarini RA, Xu T. Science. 2003;302:1227. doi: 10.1126/science.1088474. [DOI] [PubMed] [Google Scholar]
- 66.Berx G, et al. EMBO J. 1995;14:6107. doi: 10.1002/j.1460-2075.1995.tb00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]